 NATIVE
RANGE NATIVE
RANGE
Japan, Korea, China, Malaysia and India
DESCRIPTION
Japanese stiltgrass, or Nepalese browntop, is an annual grass with a sprawling habit. It germinates in spring and grows slowly through the summer months, ultimately reaching heights of 2 to 3½ ft. The leaves are pale green, lance-shaped, asymmetrical, 1 to 3 in. long, and have a distinctive shiny midrib. Slender stalks of tiny flowers are produced in late summer (August through September-early October) and dry fruits called achenes are produced soon afterwards.
ECOLOGICAL THREAT
Japanese stiltgrass is especially well adapted to low light conditions. It threatens native plants and natural habitats in open to shady, and moist to dry locations. Stiltgrass spreads to form extensive patches, displacing native species that are not able to compete with it. Where white-tail deer are over-abundant, they may facilitate its invasion by feeding on native plant species and avoiding stiltgrass. Japanese stiltgrass may impact other plants by changing soil chemistry and shading other plants. The interaction between stiltgrass and the Northern Pearly Eye (Enodia anthedon), a member of the brush-footed butterfly family Nymphalidae, is unclear. This butterfly is rare to uncommon along the Potomac River in the Washington, DC area. Its caterpillar eats grasses. Dr. Robert Robbins, a Smithsonian entomologist and butterfly specialist takes weekly walks at Great Falls, Maryland, and made the following observations. The Northern Pearly Eye occurs uncommonly at Great Falls from May to October (maybe 2-15 individuals seen over the entire flight period). Adults were especially common during the summer of 2004. The butterfly became exceedingly common during the summer of 2005 when about 20 adults were seen during a 2 hour walk, especially in the vicinity of stiltgrass, on which a female was observed placing an egg. In May 2006, the butterfly was again common, but the population then crashed, and only 2-3 individuals were seen from June to October 2006. Further investigation is needed to study the potential impacts of stiltgrass on this and possibly other butterflies or other insects that utilize stiltgrass as an alternative host plant.
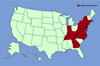 DISTRIBUTION
IN THE UNITED STATES DISTRIBUTION
IN THE UNITED STATES
According to the WeedUS Database, Japanese stiltgrass has been reported to be invasive in natural areas in 15 eastern states inculding Connecticut, Delaware, Georgia, Indiana, Kentucky, Maryland, Massachusetts, New Jersey, New York, North Carolina, Pennsylvania, Tennessee, Virginia, West Virginia, and Washington, DC.
 HABITAT
IN THE UNITED STATES HABITAT
IN THE UNITED STATES
Stiltgrass occurs in a wide variety of habitats including moist ground of open woods, floodplain forests, wetlands, uplands, fields, thickets, paths, clearings, roadsides, ditches, utility corridors, and gardens. It readily invades areas subject to regular mowing, tilling, foot traffic, and other soil disturbing activities as well as natural disturbances such as the scouring associated with flooding. Stiltgrass appears to prefer moist, acidic to neutral soils that are high in nitrogen.
BACKGROUND
First documented in Tennessee around 1919, stiltgrass may have accidentally escaped as a result of its use as a packing material for porcelain.
BIOLOGY & SPREAD
Japanese stiltgrass is an annual grass, with all plants dying each fall. It is a colonial species that spreads during the summer and fall by rooting at stem nodes that touch the ground. Individual plants may produce 100 to 1,000 seeds that fall close to the parent plant from both self-fertilizing and cross-fertilizing flowers. Seed may be carried further by water currents during heavy rains or moved in contaminated hay, soil, or potted plants, and on footwear and vehicles. Stiltgrass seed remains viable in the soil for five or more years and germinates readily. Deer and other grazers reportedly do not browse it, though they have been found to spread the seeds. Stiltgrass leaves a thick layer of thatch after dieback each year in heavily invaded areas, and while leaves decompose quickly, stems do not. Like other invasive species, stiltgrass is physiologically adaptive. For example, it is able to withstand low light levels where nutrient levels are sufficient, and able to withstand low nutrient levels where light levels are sufficient. While stiltgrass can photosynthesize in low light conditions and respond quickly to the changing light conditions typically found on the forest floor, the very low light conditions found beneath a multilayered forest canopy will limit its growth.
MANAGEMENT OPTIONS
A variety of control methods are available for stiltgrass, depending on the extent of the infestation, the type of habitat, and the availability of labor and other resources. Preventing the introduction of stiltgrass from infested to non-infested areas should be a priority. Early control of new infestations will also reduce the likelihood of establishment and expansion. Manual removal of plants results in unavoidable disturbance to the soil which can result in additional germination of stiltgrass seed. Using an herbicide leaves the plants and soil in place, thus minimizing that likelihood.
Biological
No biological controls are currently available for this plant.
Chemical
For extensive stiltgrass infestations, use of a systemic herbicide such as glyphosate (e.g., Roundup Pro®) is a practical and effective method if used with some caution. Glyphosate is a non-specific herbicide that will kill or damage almost any herbaceous plant and possibly some woody plants it contacts. Roundup Pro® is surfactant-loaded (no additional surfactant needed) and the surfactant is not lethal to amphibians and aquatic invertebrates like the polyoxyethyleneamine surfactant in Roundup Classic® is. Roundup Pro® carries the 'Caution' signal word while Roundup Classic® carries 'Warning'. When treating stiltgrass in wetland sites, use Rodeo® or other formulation labeled for wetlands. Apply a 2% solution of Roundup® or Rodeo® mixed with water (8 oz. per 3 gals. mix) and a surfactant in late summer. Be careful to avoid application to non-target plants.
Some researchers have also found success using the pre-emergent herbicide imazapic which is the active ingredient found in Plateau® (for government use only), and Journey® (for all other applicators). Imazapic is most effective against stiltgrass when applied in March in the Mid-Atlantic states. The best rate for maximum selectivity is 4 oz. per acre, applied as a broadcast application with backpack sprayers. Sprayers should be fitted with an 8003E flat fan nozzle and calibrated at 15 to 20 gpa. Plateau® and Journey® can be applied continually through germination of the stiltgrass and throughout the summer during its peak growth. No surfactant is necessary for pre-emergent applications. As germination nears, begin to add 1/4% non-ionic surfactant to the mixture.
Another option that may be appropriate for certain situations is to apply a pre-emergent (only) treatment with Pendulum® Aquacap™ (active ingredient is pendimethalin) at 2.4 qts. to 4.8 qts. per acre (15 to 20 gpa). The higher rates have provided season long control.
Note: Calibration of spray equipment will ensure that the correct rate of herbicide mix is actually applied to the plants. Actual rate of application can vary widely based on different skills and techniques of applicators. These differences can lead to under-application or over-application of herbicide mix which can affect the efficacy of the treatment. For this reason, it is important to calibrate spray equipment before conducting herbicide applications.
Manual
Stiltgrass is a shallow-rooted annual that can be pulled by hand throughout the growing season, especially when the soil is moist and entire plants with roots can be removed. Pulling is easier and probably more effective in mid-to-late summer when the plants are much taller and more branched. At this stage, entire plants can be easily removed by grabbing the basal portion of a plant and pulling firmly. In short time, a fair amount of stiltgrass can be pulled and piled up to dehydrate on site. If plants are already in the fruiting stage, they should be bagged and disposed of offsite to prevent dispersal of seed. Also, try to avoid pulling native grasses like Virginia cutgrass (Leersia virginia) that often grow intermingled with stiltgrass and may be difficult to distinguish from it. Because hand pulling plants disturbs the soil and may expose stiltgrass seed from previous seasons, late season pulling will avoid the likelihood of seed germination. Hand pulling of plants will need to repeated and continued for many seasons until the seed bank is exhausted.
Mechanical
Stiltgrass can be mowed in late summer (i.e., August through September) when the plants are flowering but preferably before seed is produced. This can be done using a lawn mower or "Weed Whacker" type machine or a scythe. Because stiltgrass is primarily an annual plant, cutting late in the season before the plants would die back naturally avoids the possibility of regrowth. Recent information suggests that stiltgrass plants that are cut early in the summer respond by regrowing and flowering soon after cutting, much earlier than they would normally flower. This is another reason to consider cutting in late summer to fall rather than during the early summer months.
USE PESTICIDES WISELY: ALWAYS READ THE ENTIRE PESTICIDE LABEL CAREFULLY, FOLLOW ALL MIXING AND APPLICATION INSTRUCTIONS AND WEAR ALL RECOMMENDED PERSONAL PROTECTIVE GEAR AND CLOTHING. CONTACT YOUR STATE DEPARTMENT OF AGRICULTURE FOR ANY ADDITIONAL PESTICIDE USE REQUIREMENTS, RESTRICTIONS OR RECOMMENDATIONS.
NOTICE: MENTION OF PESTICIDE PRODUCTS ON THIS WEB SITE DOES NOT CONSTITUTE ENDORSEMENT OF ANY MATERIAL.
CONTACTS
For more information on the management
of Japanese stiltgrass, please contact:
- Art Gover, PENNDOT Roadside Vegetation Management Project, Department of Horticulture, The Pennsylvania State University, University Park, PA; (814) 863-1184 phone/fax; aeg2(at)psu.edu
- Fred Yelverton, North Carolina State University, Raleigh, NC; (919) 515-5639; Fred_Yelverton(at)ncsu.edu
- Joseph C. Neal, North Carolina State University, Raleigh, NC; joe_neal(at)ncsu.edu
- Jeffrey F. Derr, Virginia Polytechnic Institute and State University, Virginia Beach, VA; jderr(at)vt.edu
OTHER LINKS
AUTHOR
Jil M. Swearingen, National Park Service, Center for Urban Ecology, Washington, DC
Sheherezade Adams, University of Maryland, Frostburg, MD
REVIEWERS
Jim Bean, BASF Corporation, Collierville, TN
Art Gover, The Pennsylvania State University, Philadelphia PA
Todd L. Mervosh, Weed Scientist, The Connecticut Agricultural Experiment Station, Windsor, CT Robert K. Robbins, Smithsonian Institution, Washington DC
PHOTOGRAPHS
Chuck Bargeron, www.invasive.org, University of Georgia, GA
REFERENCES
Barden, Lawrence. 1987. Invasion
of Microstegium vimineum (Poaceae), an exotic, annual, shade-tolerant,
C-4 grass, into a North Carolina floodplain. The American Midland Naturalist
118 (1):40-45.
Barden, Lawrence. 1991. Element
Stewardship Abstract: Microstegium vimineum. The Nature Conservancy.
Claridge, K and Franklin, SB. 2003. Compensation and plasticity in an invasive plant species. Biological Invasions 4: 339-347.
Cole, PG and Weltzin, JF. 2004. Environmental correlates of the distribution and abundance of Microstegium vimineum, in east Tennessee. Southeastern Naturalist 3: 545-562.
Cole, PG and Weltzin, JF. 2005. Light limitation creates patchy distribution of an invasive grass in eastern deciduous forests. Biological Invasions 7: 477-488.
Ehrenfeld, JG, Kourtev, P and Huang, W. 2001. Changes in soil functions following invasions of exotic understory plants in deciduous forests. Ecological applications 11: 1287-1300.
Fairbrothers, D. E. and J.R. Gray.
1972. Microstegium vimineum (Trin.) A. Camus (Gramineae) in
the United States. Bulletin of the Torrey Botanical Club 99:97-100.
Horton, JL and Neufeld, HS. 1998. Photosynthetic responses of Microstegium vimineum (Trin.) A. Camus, a shade-tolerant, C4 grass, to variable light environments. Oecologia 114: 11-19.
Hunt, D. M. and Robert E. Zaremba.
1992. The northeastward spread of Microstegium vimineum (Poaceae)
into New York and adjacent states. Rhodora 94:167- 170.
LaFleur, A. 1996. Invasive plant
information sheet: Japanese stiltgrass. The Nature Conservancy, Connecticut
Chapter Connecticut, Hartford, CT.
Mehrhoff, LJ. 2000. Perennial Microstegium vimineum (Poaceae): an apparent misidentification? Journal of the Torrey Botanical Society 127: 251-254.
Miller, J.H. 2003. Nonnative invasive
plants of southern forests: a field guide for identification and control.
Gen. Tech. Rep. SRS-62. Asheville, NC: U.S. Department of Agriculture, Forest
Service, Southern Research Station. 93 pp.
Redman, Donnell E. 1995. Distribution
and habitat types for Nepal microstegium [Microstegium vimineum (Trin.)
Camus] in Maryland and the District of Columbia. Castanea 60(3): 270-275.
Rhoads, A.F. and T.A Block. 2000.The
Plants of Pennsylvania, An Illustrated Manual. University of Pennsylvania
Press. 1061 pp.
Swearingen, J. 2009. WeedUS Database of Plants Invading Natural Areas in the United States: Japanese Stiltgrass (Microstegium vimineum). http://www.invasive.org/weedus/subject.html?sub=3051.
USDA, NRCS. 2009. The PLANTS Database (http://plants.usda.gov). National Plant Data Center, Baton Rouge, LA 70874-4490 USA.
Winter, K, Schmitt, MR and Edwards, GE. 1982. Microstegium vimineum, a shade adapted C-4 grass. Plant Science Letters 24: 311-318.
Plant Conservation Alliance, Alien Plant Working Group.
|


 NATIVE
RANGE
NATIVE
RANGE
 HABITAT
IN THE UNITED STATES
HABITAT
IN THE UNITED STATES