|
Baseline Glycogen Levels for Elliptio mcmichaeli (Clench and Turner, 1956); Seasonality Between two sites in the Choctawhatchee River Watershed and Laboratory Holding
Jeffrey J. Herod, Holly N. Blalock-Herod,
D. Shane Ruessler, and James D. Williams
U.S. Geological Survey, Florida Integrated Science Center
7920 NW 71st Street, Gainesville, FL 32653
Presented to the Freshwater Mollusks Conservation Society. March 12-14, 2001. Pittsburgh, PA.
Abstract
Describing Elliptio mcmichaeli population dynamics at two sites within the Choctawhatchee River Watershed includes demographical data for shell parameters, weights of soft tissue, and seasonality of glycogen. A total of 120 Elliptio mcmichaeli, 20 each from the Choctawhatchee River proper and the Pea River for the spring, fall, and holding experiment, were used in glycogen analysis to examine variations within and between populations, and over time. Mussels used in the glycogen analysis were measured and weighed. The fall field and captive samples were compared for both the Choctawhatchee and Pea rivers. Comparison of field and captive samples for the Choctawhatchee River reveal shell length was not significantly different, but the glycogen levels were significantly different. Comparison of field and captive samples for the Pea River found shell length was not significantly different, but the glycogen levels were significantly different. Glycogen concentrations after laboratory holding for 5 months were not significantly different between the two populations. These data are being included into the long-term study involving comparative analysis of Elliptio mcmichaeli from two river systems.
Holding in Aquariums
Individuals from the May sample were placed in aquariums for holding as part of glycogen comparisons. Twenty mussels from each collection were chosen at random for glycogen analysis. Mussels from HNB99-33 collected on May 20, 1999 were sacrificed on 20 October 1999. Mussels from HNB99-34 collected on 20 May 1999 were sacrificed on 21 October 1999. Whole wet weight was measured with the water and bodies still in the shell. The adductor mussels were cut with an oyster knife to open and drain the shell. The mantle tissue was cut from the rest of the body and scraped off the shell and placed on Chem Wipe. The body was then removed and placed on the Chem Wipe. The wet shell was weighed again, the nacre was then dried with a towel and labeled with a sharpie. The tissues were blotted to remove excess water and were then weighed for a total wet body weight. The mantle was then weighed separately for a wet mantle weight. The mantle was then put in a plastic tube and frozen at –11 °C until analysis. The remainder of the tissue was discarded. Shells were allowed to air dry.
Statistical Analysis of Shell and Tissue Data
Comparing shell and tissue morphometry and glycogen concentrations of Elliptio mcmichaeli within a site (CR and PR) among seasons, single factor ANOVA was utilized in Microsoft Excel. Seasons were Spring (May sample), Fall (November sample), and the holding trial (October sacrifice of samples held in the laboratory for approximately 5 months). Significance was based on a value of p < 0.05.
Glycogen Methods
A total of 120 Elliptio mcmichaeli (20 from each site and each time period: spring, fall, and holding experiment) were used in the glycogen analysis to examine variations within and between populations, and over time. Mussels used in the glycogen analysis were measured (length, width, height) and weighed (whole wet weight, wet shell weight, dry shell weight) as described above (in baseline population analysis). Mussels were dissected to remove all visceral mass and blotted with a Chem Wipe to remove excess water. Whole wet tissue was weighed on a Ohaus CT 200 scale. Next, the entire mantle tissue was removed from the visceral mass, weighed, placed in 2 mL screw cap cryovials and immediately frozen (-80 °C) until analysis.
Digestion and assay methodology are modified from Naimo et al, 1998. Digestion procedures began by adding 30% aqueous KOH (w/v) to each sample at a rate of 3 times the tissue weight. Samples were then heated, in shaking water bath at 100 °C, for 20 minutes. After removal from the water bath, samples were vortexed for 30 seconds and chilled on ice for 5 minutes. After cooling, 200 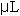 of the digested mantle KOH solution and 200 of the digested mantle KOH solution and 200 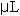 of 95% ethanol was pipetted into 6 mL snaptop scintillation vials. (The remainder of mantle solution sample was discarded). The scintillation vials were then vortexed for 5 seconds and placed into a shaking water bath at 100 °C for 15 minutes. After removing each scintillation vials from heat, 1.2 ml of deionized water was added to each sample. Each sample was then vortexed for 10 seconds and allowed to stand at room temperature for 5 minutes. Following the digestion procedure the samples were ready to be assayed. of 95% ethanol was pipetted into 6 mL snaptop scintillation vials. (The remainder of mantle solution sample was discarded). The scintillation vials were then vortexed for 5 seconds and placed into a shaking water bath at 100 °C for 15 minutes. After removing each scintillation vials from heat, 1.2 ml of deionized water was added to each sample. Each sample was then vortexed for 10 seconds and allowed to stand at room temperature for 5 minutes. Following the digestion procedure the samples were ready to be assayed.
Glycogen standards were made from commercially available, Type VII Glycogen from the blue mussel, Mytilus edulis. A serial dilution of a single high concentration served as the calibration curve. The high concentration standard was prepared diluting 200 mg of glycogen with deionized water in a volumetric flask (2000 mg/L concentration). Glycogen standards were as follows: 2000 mg/L, 1000 mg/L, 500 mg/L, 250 mg/L, 125 mg/L, and a control, (0 mg/L). Glycogen standard concentrations were adjusted for tissue mass and all results reported as the concentration of glycogen per gram tissue.
The assay procedure was microtized from Naimo, et al (1998) to increase productivity and accuracy. Microwell plates with 96 individual well volumes of 450 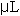 each were used. Three replicates of 25 each were used. Three replicates of 25 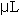 of each standard and each sample were pipetted in into individual wells of a well plate. Next, 10 of each standard and each sample were pipetted in into individual wells of a well plate. Next, 10 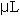 of 80% aqueous phenol (v/v) and 200 of 80% aqueous phenol (v/v) and 200 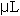 of reagent grade H2SO4 were added to each standard and sample in the plate. The microwell plate was covered, allowed to settle at room temperature for 5-10 minutes, and then placed in a Dynex MRX Microplate Reader. All standards and samples were read in triplicate. Data output was recorded in mg/g tissue weight and was graphed in Microsoft Excel. All statistical analyses were conducted in SAS as described above (SAS, 1985). of reagent grade H2SO4 were added to each standard and sample in the plate. The microwell plate was covered, allowed to settle at room temperature for 5-10 minutes, and then placed in a Dynex MRX Microplate Reader. All standards and samples were read in triplicate. Data output was recorded in mg/g tissue weight and was graphed in Microsoft Excel. All statistical analyses were conducted in SAS as described above (SAS, 1985).
|