For Consumers
FDA Works to Lessen Drug Shortage Impact
|
|
 Get Consumer Updates by E-mail
Get Consumer Updates by E-mail
 Share copies of this article (598 KB)
Share copies of this article (598 KB)
Federal regulators are asking U.S. drug makers for advance warning of production shortages, saying medicine scarcities are continuing to increase at a rapid pace after reaching a record high in 2010.
Food and Drug Administration (FDA) officials are working closely with industry, health care providers, and patients to prevent and mitigate shortages of “medically necessary” medicines. Medically necessary drugs are those used to treat or prevent a serious disease or medical condition for which there is no alternative medicine available in adequate supply.
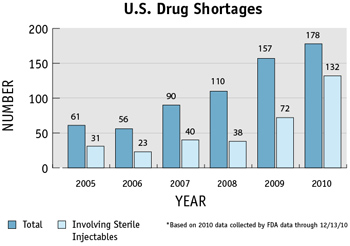
FDA says the number of drug shortages has nearly tripled over the last six years—jumping from 61 drug products in 2005 to 178 in 2010—and that doesn’t include shortages of vaccines, immune globulin products, and other biologics, or products made from blood, tissue, or other biological source.
FDA’s Valerie Jensen, a pharmacist and expert on drug shortages, says most of the supply problems have involved “sterile injectables”—medicines given by injection or intravenously to patients in the hospital. The shortages involve cancer drugs; anesthetics used for patients having surgery; “crash cart” drugs used in emergencies; electrolytes for patients being fed intravenously; and other drug products.
What to Do?
There have also been shortages of prescription medicines that many Americans take by mouth each day, including some drugs prescribed for Attention Deficit Hyperactivity Disorder (ADHD).
“FDA is doing everything within its regulatory authority to address these shortages when they occur,” Jensen says.
If a drug you’re taking is not available and you believe it might be in short supply:
- Visit FDA’s Current Drug Shortages page to see if your drug has been reported as being in short supply.
- If your drug isn’t on the FDA’s list, send the name and dose of the drug and your contact information to FDA’s Center for Drug Evaluation and Research at drugshortages@fda.hhs.gov.
- If your medicine is in short supply, talk with your doctor. There may be other medicines available that can be prescribed for your illness.
FDA's Role
During 2010, quality and manufacturing problems were to blame for many of the shortages in the United States, says Ilisa Bernstein, deputy director of compliance in FDA’s Center for Drug Evaluation and Research.
Shortages were fueled by many different factors, including a lack of raw materials used in the manufacturing process; increased demand for some drugs; and a company’s business decision to stop making some older, less profitable drugs.
While FDA encourages companies to notify the agency about problems that could lead to shortages, Bernstein says there is currently no legal requirement for them to tell FDA. Companies that are the sole source of a medically necessary drug are legally required to inform FDA six months in advance if they plan to discontinue making that product; however, there is no legal penalty if they choose not to do so.
“FDA is urging drug makers to voluntarily notify us if they change production quantities of drugs as a matter of corporate responsibility and in the interest of public health,” Bernstein says.
When the agency gets advance warning that a shortage could occur, FDA works with other firms that make the drug and asks if they can ramp up production to fill any gap in the domestic supply of the product.
In certain situations, when manufacturers of an FDA-approved drug can't immediately resolve a shortage of a medically necessary drug, FDA sometimes identifies foreign versions of the product with the same active ingredient manufactured by reputable firms. FDA then uses enforcement discretion for the limited importation of the foreign version until the shortage of the FDA-approved drug is resolved.
Last year, FDA was able to help prevent 38 drug shortages because companies notified the agency of issues that could lead to supply disruptions.
This article appears on FDA's Consumer Updates page, which features the latest on all FDA-regulated products.
June 9, 2011