Medical Devices
Unique Device Identification
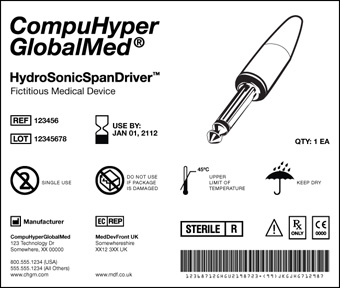
Example of what a universal device identifier (UDI) would look like on a medical device label. The label contains information about the product name, its expiration date, reference and lot numbers, manufacturer information, bar code, details about the item, and an illustration of the item. (View larger image.)
The Food and Drug Administration (FDA) has released a proposed rule that most medical devices distributed in the United States carry a unique device identifier, or UDI. Congress passed legislation in 2007 directing the FDA to develop regulations establishing a unique device identification system for medical devices.
A UDI system has the potential to improve the quality of information in medical device adverse event reports, which will help the FDA identify product problems more quickly, better target recalls and improve patient safety.
A UDI is a unique numeric or alphanumeric code that includes a device identifier, which is specific to a device model, and a production identifier, which includes the current production information for that specific device, such as the lot or batch number, the serial number and/or expiration date.
The FDA is also creating a database that will include a standard set of basic identifying elements for each UDI, and will make most of it available to the public so that users of a medical device can easily look up information about the device. The UDI does not indicate and FDA’s database will not contain any information about who uses a device, including personal privacy information.
In developing the proposed rule, the FDA worked closely with industry, the clinical community and patient and consumer groups, and conducted four pilot studies.
Read the Unique Device Identifier Proposed Rule here.
Benefits of Unique Device Identification
When fully implemented, the UDI system may:
- Allow more accurate reporting, reviewing and analyzing of adverse event reports so that problem devices can be identified and corrected more quickly.
- Reduce medical errors by enabling health care professionals and others to more rapidly and precisely identify a device and obtain important information concerning the characteristics of the device.
- Enhance our analysis of devices on the market by providing a standard and clear way to document device use in electronic health records, clinical information systems, claim data sources and registries. A more robust postmarket surveillance system can also be leveraged to support premarket approval or clearance of new devices and new uses of currently marketed devices.
- Provide a standardized identifier that will allow manufacturers, distributors and healthcare facilities to more effectively manage medical device recalls.
- Provide a foundation for a global, secure distribution chain, helping to address counterfeiting and diversion and prepare for medical emergencies.
- Lead to the development of a medical device identification system that is recognized around the world.
Contact Us
For further information contact:
Jay Crowley
Center for Devices and Radiological Health
Food and Drug Administration
Phone: 301-796-5995
e-mail: Jay.Crowley@fda.hhs.gov
UDI Piloting Activities
- Unique Device Identifier Proposed Rule: July 10, 2012
- Press Release: FDA proposes unique device identification system for medical devices
- Results of FDA Pilot Activities To Explore Opportunities and Challenges With the Implementation of a Unique Device Identifier System, November 30, 2010
- SSS-GHX Development of the Prototype Unique Device Identifier Database: Report of a Pilot Test on Usability and Feasibility, November 20, 2009
UDI Related Trainings, Papers, and Reports
- CDRH Learn Online Video Presentation with Captioning (English)
Global Harmonization Task Force Reports 
- ERG Final Report: Unique Identification for Medical Devices, March 22, 2006
- Report on Meeting to Discuss Unique Device Identification, October 27, 2005
- Task 4 White Paper - Automatic Identification of Medical Devices - Final Version, August 17, 2005
- Report on Meeting to Discuss Unique Device Identification, April 14-15, 2005
UDI Public Meetings and Notices
- Materials from the FDA’s Public Workshop on Unique Device Identification (UDI) for Postmarket Surveillance and Compliance, September 12-13, 2011
- FDA Unique Device Identification Public Workshop, February 12, 2009
- Comments Received from the August 9, 2006 Public Notice on Unique Device Identification, November 9, 2006
- FDA Public Meeting on Unique Device Identification, October 25, 2006
- Public Notice that requested comments on Unique Device Identification, August 9, 2006