NIDDK FY2010 President’s Budget Request
NATIONAL INSTITUTES OF HEALTH
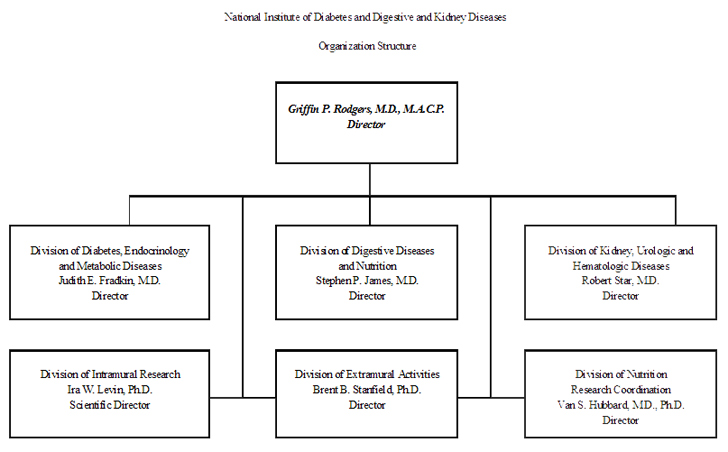
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
FY 2010 Budget
Print Version (PDF, 968KB 27pgs)
For carrying out section 301 and title IV of the Public Health Service Act with respect to diabetes and digestive and kidney disease [$1,761,338,000] $1,781,494,000 (Department of Health and Human Services Appropriation Act, 2009)
Amounts Available for Obligation1
| Source of Funding | FY 2008 (Actual) | FY 2009 (Estimate) | FY 2010 (PB) |
|---|
Appropriation | $1,736,199,000 | $1,761,338,000 | $1,781,494,000 |
| Type 1 Diabetes2 | 150,000,000 | 150,000,000 | 150,000,000 |
| Rescission | -30,331,000 | 0 | 0 |
| Supplemental | 9,077,000 | 0 | 0 |
| Subtotal, adjusted appropriation | 1,864,945,000 | 1,911,338,000 | 1,931,494,000 |
| Real transfer under Director's one-percent transfer authority (GEI) | -2,724,000 | 0 | 0 |
| Comparative transfer from NHLBI for administration of NIBIB's Director's laboratory | 816,000 | 0 | 0 |
| Comparative transfer under Director's one-percent transfer authority (GEI) | 2,724,000 | 0 | 0 |
| Subtotal, adjusted budget authority | 1,865,761,000 | 1,911,338,000 | 1,931,494,000 |
| Unobligated balance lapsing | -33,000 | 0 | 0 |
| Total obligations | 1,865,728,000 | 1,911,338,000 | 1,931,494,000 |
1 Excludes the following amounts for reimbursable activities carried our by this account;
FY2008 - $9,204,000 FY2009 Estimate - $12,000,000 FY2010 Estimate - $12,000,000
Excludes $3,965,000 Actual in FY2008; $5,000,000 Extimate in FY2009 and $5,000,000 in FY2010 for royalties.
2 Type 1 Diabetes Special Statutory Authority in Accordance with P.L. 107-360 and P.L. 110-275.
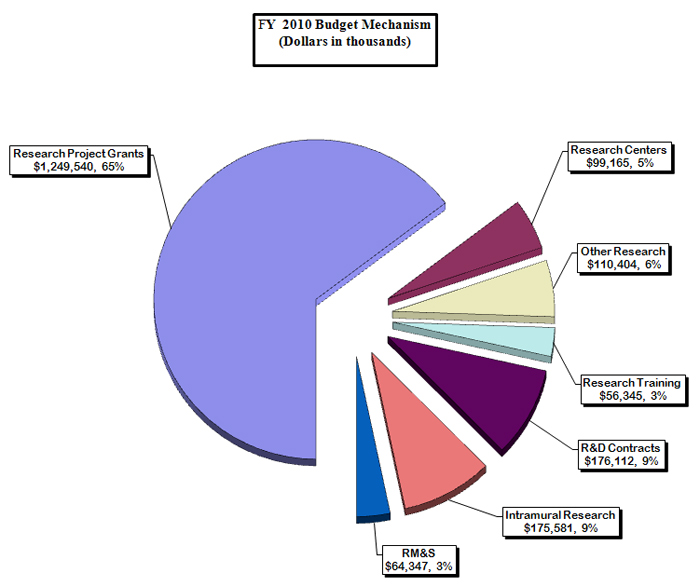
NIDDK Budget Mechanism - Total
(Dollars in Thousands) | MECHANISM | FY 2008 (Actual) No. | FY 2008 (Actual)Amount | FY 2009 (Estimate)No. | FY 2009 (Estimate)Amount | FY 2010 (PB)No. | FY 2010 (PB)Amount | ChangeNo. | Change Amount |
|---|
| Research Grants: | | | | | | | | |
|---|
| Research Projects: | | | | | | | | |
| Noncompeting | 2,210 | $859,065 | 2,135 | $861,383 | 2,172 | $862,453 | 37 | $1,070 |
| Administrative supplements | (113) | 20,185 | (183) | 30,185 | (183) | 30,185 | (0) | 0 |
| Competing: | | | | | | | | |
| Renewal | 273 | 114,433 | 288 | 126,242 | 303 | 131,137 | 15 | 4,895 |
| New | 435 | 155,938 | 471 | 171,869 | 484 | 178,824 | 13 | 6,955 |
| Supplements | 2 | 272 | 2 | 300 | 2 | 300 | 0 | 0 |
| Subtotal, competing | 710 | 270,643 | 761 | 298,411 | 789 | 310,261 | 28 | 11,850 |
| Subtotal, RPGs | 2,920 | 1,149,893 | 2,896 | 1,189,979 | 2,961 | 1,202,899 | 65 | 12,920 |
| SBIR/STTR | 106 | 45,399 | 109 | 46,145 | 110 | 46,641 | 1 | 496 |
| Subtotal, RPGs | 3,026 | 1,195,292 | 3,005 | 1,236,124 | 3,071 | 1,249,540 | 66 | 13,416 |
| Research Centers | | | | | | | | |
| Specialized/comprehensive | 79 | 90,552 | 82 | 92,725 | 82 | 94,116 | 0 | 1,391 |
| Clinical research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biotechnology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative medicine | 0 | 4,807 | 0 | 5,049 | 0 | 5,049 | 0 | 0 |
| Research Centers in Minority Institutions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subtotal, Centers | 79 | 95,359 | 82 | 97,774 | 82 | 99,165 | 0 | 1,391 |
| Other Research | | | | | | | | |
| Research careers | 526 | 71,086 | 535 | 72,302 | 535 | 72,302 | 0 | 0 |
| Cancer education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cooperative clinical research | 0 | 2,983 | 0 | 3,500 | 0 | 3,500 | 0 | 0 |
| Biomedical research support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minority biomedical research support | 0 | 54 | 0 | 54 | 0 | 54 | 0 | 0 |
| Other | 87 | 33,273 | 87 | 34,051 | 87 | 34,548 | 0 | 497 |
| Subtotal, Other Research | 613 | 107,396 | 622 | 109,907 | 622 | 110,404 | 0 | 497 |
| Total Research Grants | 3,718 | 1,398,047 | 3,709 | 1,443,805 | 3,775 | 1,459,109 | 66 | 15,304 |
| | | | | | | | |
| Research Training: | FTTPs | FTTPs | FTTPs | FTTPs | FTTPs | FTTPs | FTTPs | FTTPs |
| Individual awards | 219 | 9,833 | 219 | 9,911 | 219 | 9,911 | 0 | 0 |
| Institutional awards | 978 | 46,070 | 978 | 46,434 | 978 | 46,434 | 0 | 0 |
| Total, Training | 1,197 | 55,903 | 1,197 | 56,345 | 1,197 | 56,345 | 0 | 0 |
| | | | | | | | |
| Research & development contracts | 285 | 181,324 | 286 | 174,961 | 288 | 176,112 | 2 | 1,151 |
| (SBIR/STTR) | 3 | 105 | (3) | (105) | (3) | (105) | (0) | (0) |
| | | | | | | | |
| | | | | | | | |
| FTEs | FTEs | FTEs | FTEs | FTEs | FTEs | FTEs | FTEs |
| Intramural research | 401 | 168,667 | 383 | 172,986 | 391 | 175,581 | 8 | 2,595 |
| Research management and support | 245 | 61,820 | 235 | 63,241 | 239 | 64,347 | 4 | 1,106 |
| Construction | | 0 | | 0 | | 0 | | 0 |
| Buildings and Facilities | | 0 | | 0 | | 0 | | 0 |
| Total, NIDDK | 646 | 1,865,761 | 618 | 1,911,338 | 630 | 1,931,494 | 12 | 20,156 |
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research
Budget Mechanism - Type 1 Diabetes Only
(Dollars in Thousands) | MECHANISM | FY 2008 (Actual)No. | FY 2008 (Actual)Amount | FY 2009 (Estimate)No. | FY 2009 (Estimate)Amount | FY 2010 (PB)No. | FY 2010 (PB)Amount | Change No. | Change Amount |
|---|
| Research Grants: | | | | | | | | |
|---|
| Research Projects: | | | | | | | | |
| Noncompeting | 60 | $30,337 | 64 | $37,925 | 67 | $40,770 | 3 | $2,845 |
| Administrative supplements | (3) | 9,348 | (3) | 9,348 | (3) | 9,348 | (0) | 0 |
| Competing: | | | | | | | | |
| Renewal | 6 | 5,169 | 6 | 5,646 | 5 | 4,778 | (1) | -868 |
| New | 10 | 23,499 | 11 | 25,669 | 9 | 21,722 | (2) | -3,947 |
| Supplements | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subtotal, competing | 16 | 28,668 | 17 | 31,315 | 14 | 26,500 | (3) | (4,815) |
| Subtotal, RPGs | 76 | 68,353 | 81 | 78,588 | 81 | 76,618 | 0 | -1,970 |
| SBIR/STTR | 7 | 4,167 | 7 | 4,167 | 7 | 4,167 | 0 | 0 |
| Subtotal, RPGs | 83 | 72,520 | 88 | 82,755 | 88 | 80,785 | 0 | -1,970 |
| Research Centers | | | | | | | | |
| Specialized/comprehensive | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Clinical research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biotechnology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative medicine | 0 | 4,758 | 0 | 5,000 | 0 | 5,000 | 0 | 0 |
| Research Centers in Minority Institutions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subtotal, Centers | 0 | 4,758 | 0 | 5,000 | 0 | 5,000 | 0 | 0 |
| Other Research | | | | | | | | |
| Research careers | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cancer education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cooperative clinical research | 0 | 2,983 | 0 | 3,500 | 0 | 3,500 | 0 | 0 |
| Biomedical research support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minority biomedical research support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 0 | 874 | 0 | 874 | 0 | 874 | 0 | 0 |
| Subtotal, Other Research | 0 | 3,857 | 0 | 4,374 | 0 | 4,374 | 0 | 0 |
| Total Research Grants | 83 | 81,135 | 88 | 92,129 | 88 | 90,159 | 0 | -1,970 |
| | | | | | | | |
| Research Training: | FTTPs | FTTPs | FTTPs | FTTPs | FTTPs | FTTPs | FTTPs | FTTPs |
| Individual awards | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Institutional awards | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total, Training | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| | | | | | | | |
| Research & development contracts | 22 | 68,278 | 15 | 57,271 | 16 | 59,231 | 1 | 1,960 |
| (SBIR/STTR) | (0) | (0) | (0) | (0) | (0) | (0) | (0) | (0) |
| | | | | | | | |
| FTEs | FTEs | FTEs | FTEs | FTEs | FTEs | FTEs | FTEs |
| Intramural research | | 0 | | 0 | | 0 | 0 | 0 |
| Research management and support | | 587 | | 600 | | 610 | 0 | 10 |
| Construction | | 0 | | 0 | | 0 | | 0 |
| Buildings and Facilities | | 0 | | 0 | | 0 | | 0 |
| Total, NIDDK | | 150,000 | | 150,000 | | 150,000 | 0 | 0 |
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research
BA by Program
(Dollars in Thousands) | Extramural Research | FY 2006 (Actual)FTEs | FY 2006 (Actual)Amount | FY 2007 (Actual)FTEs | FY 2007 (Actual)Amount | FY 2008 (Actual)FTEs | FY 2008 (Actual)Amount | FY 2008 (Comparable)FTEs | FY 2008 (Comparable)Amount | FY 2009 (Estimate)FTEs | FY 2009 (Estimate)Amount | FY 2010 (PB)FTEs | FY 2010 (PB) Amount | Change FTEs | Change Amount |
|---|
| Detail: | | | | | | | | | | | | | | |
|---|
| Diabetes, Endocrinology, and Metabolic Diseases | | $652,641 | | $630,171 | | $613,897 | | $615,074 | | $625,542 | | $631,752 | | 6,210 |
| Digestive Diseases and Nutrition | | 428,918 | | 430,187 | | 442,143 | | 443,005 | | 457,713 | | 462,928 | | 5,215 |
| Kidney, Urologic, and Hematologic Diseases | | 398,966 | | 420,746 | | 426,916 | | 427,782 | | 442,456 | | 447,496 | | 5,040 |
| Type 1 Diabetes | | 150,000 | | 150,000 | | 150,000 | | 150,000 | | 150,000 | | 150,000 | | |
| Subtotal, Extramural | | 1,630,525 | | 1,631,104 | | 1,632,956 | | 1,635,861 | | 1,675,711 | | 1,692,176 | | 16,465 |
| Intramural research | 428 | 164,232 | 414 | 164,008 | 401 | 167,851 | 401 | 168,667 | 383 | 172,986 | 391 | 175,581 | 8 | 2,595 |
| Res. management & support | 210 | 58,996 | 222 | 57,884 | 245 | 61,381 | 245 | 61,233 | 235 | 62,641 | 239 | 63,737 | 4 | 1,096 |
| TOTAL | 638 | 1,853,753 | 636 | 1,852,996 | 646 | 1,862,188 | 646 | 1,865,761 | 618 | 1,911,338 | 630 | 1,931,494 | 12 | 20,156 |
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research
Major Changes in the Fiscal Year 2010 Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2010 budget request for NIDDK, which is $20.156 million more than the FY 2009 Estimate, for a total of $1,931.494 million.
Gastroparesis Clinical Research Consortium: Pilot Study for Optimal Glycemic Control of Diabetic Gastroparesis (+$0.7 million; total $4.7 million): This consortium will expand in FY 2009 and FY 2010 to conduct this special study.
Systolic Blood Pressure Intervention Trial (+$1.95 million; total $2.5 million): NIDDK is providing collaborative support to this new NHLBI-led trial; the FY 2010 funds will support initiation and development of the study protocol.
HEALTHY Study (-$7.5 million; total $2.0 million): For this school-based study of ways to reduce risk factors for type 2 diabetes, the intervention will be completed during FY 2009; follow-up data analysis and archiving of study data for NIDDK central repository in FY 2010 will be less expensive.
Collaborative Interdisciplinary Team Science in Diabetes, Endocrinology and Metabolic Diseases (R24) (+$1.0 million; total $7.0 million): FY 2010 funds will support expansion of support for new grants under this mechanism, which is being used to foster innovative approaches in inter- and trans-disciplinary research on complex biomedical research problems.
Summary of Changes
FY 2009 estimate - $1,911,338,000
FY 2010 estimated budget authority - $1,931,494,000
Net change - $20,156,000
| CHANGES | 2009 Current
Estimate Base No. | 2009 Current
Estimate Base Budget
Authority | Change from Base No. | Change from Base Budget
Authority |
|---|
A. Built-in:
1. Intramural research: | | | | |
| a. Annualization of January 2009 pay increase | | $69,388,000 | | $662,000 |
| b. January FY 2010 pay increase | | 69,388,000 | | 831,000 |
| c. Zero less days of pay | | 69,388,000 | | 0 |
| d. Payment for centrally furnished services | | 29,959,000 | | 599,000 |
| e. Increased cost of laboratory supplies, materials, and other expenses | | 73,639,000 | | 1,209,000 |
| Subtotal | | | | 3,301,000 |
| | | | |
2. Research management and support:
| | | | |
| a. Annualization of January 2009 pay increase | | $32,593,000 | | $379,000 |
| b. January FY 2010 pay increase | | 32,593,000 | | 475,000 |
| c. Zero less days of pay | | 32,593,000 | | 0 |
| d. Payment for centrally furnished services | | 2,879,000 | | 58,000 |
| e. Increased cost of laboratory supplies, materials, and other expenses | | 27,769,000 | | 469,000 |
| Subtotal | | | | 1,381,000 |
| Subtotal, Built-in | | | | 4,682,000
|
| | | | |
| B. Program: 1. Research project grants: | | | | |
| a. Noncompeting | 2,135 | $891,568,000 | 37 | $1,070,000 |
| b. Competing | 761 | 298,411,000 | 28 | 11,850,000 |
| c. SBIR/STTR | 109 | 46,145,000 | 1 | 496,000 |
| Total | 3,005 | 1,236,124,000 | 66 | 13,416,000 |
| 2. Research centers | 82 | 97,774,000 | 0 | 1,391,000 |
| 3. Other research | 622 | 109,907,000 | 0 | 497,000 |
| 4. Research training | 1,197 | 56,345,000 | 0 | 0 |
| 5. Research and development contracts | 286 | 174,961,000 | 2 | 1,151,000 |
| Subtotal, extramural | | | | 16,455,000 |
| 6. Intramural research | FTEs 383 |
172,986,000 | FTEs 8 |
(706,000) |
| 7. Research management and support | FTEs 235 |
63,241,000 | FTEs 4 |
(275,000) |
| 8. Construction | | 0 | | 0 |
| 9. Buildings and Facilities | | 0 | | 0 |
| Subtotal, program | | 1,911,338,000 | | 15,474,000 |
| Total changes | 618 | | 12 | 20,156,000 |
History of Budget Authority and FTEs:
Distribution by Mechanism:
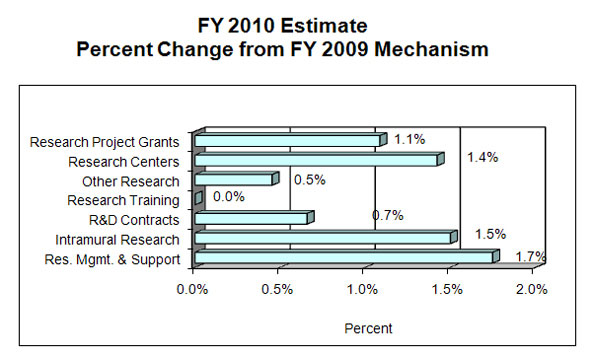
Percent Change by Mechanism:
Justification of Budget Request National Institute of Diabetes and Digestive and Kidney Diseases
Authorizing Legislation: Section 301 and title IV of the Public Health Service Act, as amended.
Budget Authority:
| FY 2008
Actual | FY 2009
Omnibus | FY 2009 Recovery Act | FY 2010
President's Budget | Increase or
Decrease FTEs | FY 2010 +/- 2009 Omnibus |
|---|
| BA | $1,864,945,000 | $1,911,338,000 | $445,393,000 | $1,931,494,000 | | +$20,156,000 |
| FTEs | 646 | 618 | | 630 | 12 | |
| Type 1 Diabetes: | -$150,000,000 | -$150,000,000 | | -$150,000,000 | | |
| Labor/HHS: | $1,714,945,000 | $1,761,338,000 | | $1,781,494,000 | | |
This document provides justification for the Fiscal Year (FY) 2010 activities of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), including HIV/AIDS activities. Details of the FY 2010 HIV/AIDS activities are in the “Office of AIDS Research (OAR)” Section of the Overview. Details on the Common Fund are located in the Overview, Volume One. Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
In FY 2009, a total of $445,393,000 American Recovery and Reinvestment Act (ARRA) funds were transferred from the Office of the Director. These funds will be used to support scientific research opportunities that help support the goals of the ARRA. The ARRA allows NIH to execute these funds via any NIH funding mechanism. Funds are available until September 30, 2010. These funds are not included in the FY 2009 Omnibus amounts reflected in this document.
DIRECTOR’S OVERVIEW
The mission of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) is to support research to combat diabetes and other endocrine and metabolic diseases, liver and other digestive diseases, nutritional disorders, obesity, kidney and urologic diseases, and hematologic diseases. These diseases are chronic, common and costly for patients, their families, and our Nation. Diabetes affects an estimated 23.6 million people in the U.S. and greatly increases the risk for many serious complications, such as heart disease and kidney failure[1] An estimated 16 million adults have moderately or severely reduced kidney function; and approximately 506,000 have irreversible kidney failure (end stage renal disease)[2] Many digestive diseases are also highly prevalent, as are urologic diseases.2,[3] Obesity affects approximately one-third of U.S. adults and about 16 percent of children and adolescents.[4] Obesity is a strong risk factor for type 2 diabetes, fatty liver disease, and many other diseases and disorders. Also within NIDDK’s research purview are cystic fibrosis and other genetic diseases which are less wide-spread, but nonetheless devastating in their impacts. Building upon the emerging opportunities that are the fruits of past research investments, the Institute will continue to pursue basic, clinical, and translational research studies; research training and career development; and health information dissemination. These efforts will lead to better strategies for predicting susceptibility to disease, preempting disease, personalizing treatments, and fostering participatory approaches, so as to improve the lives of patients, their families, and those at risk.
Genetic factors contributing to chronic disease represent an important area of emerging opportunity. Recent advances have greatly increased knowledge of genes associated with diabetes, kidney disease, and inflammatory bowel disease. NIDDK is continuing support for existing genetics consortia and planning efforts to capitalize on genetic findings to accelerate the development of new therapeutic approaches.
Epidemic levels of obesity in the U.S. are contributing to an escalation in the prevalence of type 2 diabetes, which, in turn, is the leading cause of kidney failure. NIDDK’s research on obesity, type 2 diabetes, and kidney disease represents a paradigm of its integrated research programs. To address the complex interplay of factors that promote excess weight gain, NIDDK continues to support a multidimensional research portfolio on obesity. For example, studies are elucidating the biologic pathways that regulate appetite and energy expenditure, with implications for future therapeutic strategies. Ongoing prevention and treatment studies include interventions in schools and other community sites in diverse populations, and research on the risks and benefits of bariatric surgery as a treatment for severe obesity. NIDDK-supported research on chronic, obesity-related conditions also includes studies of treatment for the “silent” but serious liver disease, nonalcoholic steatohepatitis in children and adults.
A number of NIDDK-funded studies are at the nexus of obesity and type 2 diabetes. For example, the middle school-based HEALTHY study is determining whether changes in school food services and physical education classes, along with activities to encourage healthy behaviors, will reduce type 2 diabetes risk factors in youth. NIDDK also supports research to explore cost-effective ways to translate findings from diabetes and obesity intervention studies to real-world clinical practice and community settings, with an emphasis on disproportionately-affected populations.
Several areas of study are also critical for those with type 1 diabetes, a form of diabetes that often strikes in childhood and adolescence, thus placing particular burden on children and their families. Research on type 1 diabetes is not only improving health outcomes of people with this disease, but also benefiting those with type 2 diabetes. For example, both forms of diabetes share the same devastating complications, so that research on preventing complications in type 1 diabetes has benefited type 2 diabetes.
The Institute is actively pursuing a range of research avenues on kidney disease. In one ongoing study of chronic kidney disease in adults, the diverse study population and presence of diabetes in many of the participants reflect the health disparities associated with kidney disease, as well as its link to diabetes. NIDDK also supports studies of acute kidney injury, chronic kidney disease in children, hemodialysis, and other areas. Translating knowledge into practice, NIDDK is working with community health centers to improve screening and management practices, to enable better identification and treatment of people with chronic kidney disease before they develop kidney failure.
These efforts are just a few examples of NIDDK’s broad spectrum of research areas that address the many chronic and debilitating diseases within its mission. The Institute also disseminates science-based health information through its Weight-control Information Network; the National Diabetes Education Program, which it co-sponsors with the Centers for Disease Control and Prevention (CDC); the National Kidney Disease Education Program; information clearinghouses; and other venues.
In planning for the future, NIDDK will continue to seek external advice from investigators, professional organizations, patient advocates, and the public. Input from the NIDDK’s National Advisory Council, Interagency Coordinating Committees, strategic planning processes, ad hoc planning groups, and scientific conferences and workshops will continue to inform resource allocation decisions. Active collaboration with other components of NIH and other federal agencies will also remain part of NIDDK planning efforts. For example, NIDDK has a leadership role in trans-NIH obesity research efforts and leads collaborative research efforts in diabetes, liver diseases, and other areas.
Ever-increasing knowledge and new technologies bring new research opportunities for alleviating and conquering the many diseases within NIDDK’s mission. The Institute’s continuing goal is to build upon these opportunities to improve public health.
FY 2010 Justification by Activity Detail
Overall Budget Policy: NIDDK will continue to support new investigators and to maintain an adequate number of competing RPGs. NIDDK is providing a 2 percent inflationary increase for non-competing and competing grants. In addition, the NIDDK has targeted a portion of the funds available for competing research project grants to support high priority projects outside of the payline, including awards to new investigators, and early stage investigators. The Institute also seeks to maintain a balance between solicitations issued to the extramural community in areas that need stimulation and funding made available to support investigator-initiated projects. Intramural Research and Research Management and Support receive increases to help cover the cost of pay and other increases.
In addition, consistent with the President’s commitment to increase funding for cancer research, and with the HHS-wide initiative on autism, the NIDDK will support research relevant to these diseases.
Program Descriptions and Accomplishments
Diabetes, Endocrinology, and Metabolic Diseases: The goals of this program are to increase understanding of diabetes and other diseases and disorders of the endocrine system and metabolism, and to develop and test potential prevention and treatment strategies. This program supports basic, clinical, and translational research, as well as research training, in the areas of type 1 and type 2 diabetes, cystic fibrosis, and other endocrine and metabolic disorders; obesity, neuroendocrinology, and energy balance; and development, metabolism, and basic biology of endocrine and metabolic tissues. Knowledge gained from research is broadly communicated to patients, health professionals, and the public through the support for the National Diabetes Information Clearinghouse and the National Diabetes Education Program.
In FY 2008, NIDDK completed the first phase of the DPP Outcomes Study to determine the long-term durability of Diabetes Prevention Program (DPP) interventions, with over 90 percent retention of participants, building upon previous DPP research that demonstrated that type 2 diabetes could be delayed or prevented with diet and exercise to achieve moderate weight loss, or with diabetes medication. Efforts to enhance approaches to translate the message of type 2 diabetes prevention and other research into clinical practice included an ongoing solicitation for diabetes and obesity prevention and control projects, plus expansion of Diabetes Research and Training Centers in 2008. NIDDK also collaborated with the Centers for Disease Control and Prevention, Indian Health Services, and Tribal Colleges and Universities to develop a K-12 diabetes-related science curriculum for Tribal schools aimed at reducing diabetes health disparities and developing increased interest in the biomedical sciences and in science careers related to diabetes among American Indian children. The curriculum development effort was completed in 2008. The HEALTHY study, a multi-center, middle school-based study of strategies for reducing risk factors for type 2 diabetes in children—launched in fall 2006—was continued in 2008. Over 6,300 students at the 42 middle schools where the study is being conducted participated in the baseline examinations, resulting in new knowledge of the prevalence of diabetes risk factors in middle school age children.
Budget Policy: The FY 2010 budget estimate for this program is $631.752 million, an increase of $6.210 million or 1.0 percent above the FY 2009 estimate. With FY 2010 resources, the NIDDK will continue major diabetes clinical trials and encourage and support development of major new investigator-initiated clinical studies. FY 2010 funds will also support research capitalizing on new opportunities to identify diabetes risk genes in minority populations and to advance progress toward developing new therapeutic approaches. NIDDK will also fund translational research in FY 2010 and support health information dissemination activities to bring scientific discoveries in diabetes and obesity to real world medical practice and other community settings. In FY 2010, NIDDK will continue an initiative encouraging collaborative, multidisciplinary research teams to work on complex biomedical problems in diabetes, endocrinology, and metabolic diseases. NIDDK will also continue funding for Centers advancing research relevant to cystic fibrosis and other genetic metabolic diseases. NIDDK plans for FY 2010 include continuing new imaging studies of brain function governing weight regulation and pursuing other efforts as part of an overall balanced research portfolio.
Portrait of a Program: Epidemiology of Diabetes Interventions and Complications (EDIC)
FY 2009 Level: $9.849 Million
FY 2010 Level: $9.014 Million
Change: -$0.835 Million
The EDIC study is a follow-up to the landmark Diabetes Control and Complications Trial (DCCT), a clinical trial in 1,441 patients with type 1 diabetes. Through long-term follow-up of the patients who participated in DCCT, EDIC is building upon findings of the earlier trial, which clearly demonstrated that the development of eye, kidney, and nerve complications of diabetes could be greatly reduced through intensive control of blood glucose levels, as compared to the standard of care at the time. The initiation of these research efforts was based on external input and recommendations. DCCT also established a measure of blood glucose control as an FDA-accepted surrogate marker for clinical trials, which led to the approval of new or improved therapies for type 1 and type 2 diabetes. Seminal insights continue to emerge from this research. For example, patients from the former intensive-control group continue to have long-term benefits compared to those in the former conventional-control group, even though in EDIC both groups have had comparable glucose control. This finding demonstrates the enduring benefits of a finite period of good blood-sugar control. Moreover, EDIC researchers have shown that intensive control lowered the risk of heart disease and stroke by about 50 percent—an effect that could not have been evaluated earlier, during the DCCT, because cardiovascular disease generally takes many years to develop. The importance of EDIC is underscored by recent results from other major diabetes clinical studies which build upon the DCCT/EDIC findings. In 2008, the United Kingdom Prospective Diabetes Study (UKPDS) showed that, in type 2 diabetes patients, early intensive blood glucose control reduces cardiovascular disease (“macrovascular” disease), just as it had found ten years earlier that the intervention reduces eye, kidney, and nerve complications (“microvascular” disease)—mirroring DCCT/EDIC findings in type 1 diabetes in the much more common type 2 diabetes. In contrast, recent results from the NIH-funded ACCORD study indicate that implementing even more aggressive blood glucose control late in the course of type 2 diabetes can be dangerous. Together, these studies are providing robust evidence for the long-term value of early intervention in diabetes and information important for personalized medicine or tailoring treatment in individual patients at various disease stages.
NIDDK has extended funding for EDIC until 2016 to continue to collect critical data from these well-characterized patients so as to provide further information on longer-term benefits of intensive glucose control, inform potential new prevention and therapeutic strategies, and facilitate future clinical research. For example, continued funding in FY 2010 and beyond will enable investigation of the role of diabetic kidney disease in the onset and progression of cardiovascular disease, as well as validation of surrogate markers for cardiovascular disease that will facilitate future trials. It will also permit EDIC researchers to evaluate the cost-effectiveness of the intervention as they assess continued prolonged benefit from a finite intervention that ended in 1993. Through these and other efforts, EDIC is expected to continue to make major contributions to the prevention and management of diabetes complications.
Digestive Diseases and Nutrition: The goals of this program are to increase understanding of digestive diseases, nutrition, and obesity, and to develop and test strategies for disease prevention and treatment. This program supports basic, clinical, and translational research, as well as research training, encompassing fundamental studies of the digestive system; disease-targeted research involving the esophagus, stomach, small intestine, large intestine and anorectum, liver and biliary system, and pancreas; studies relevant to nutrition; and research on obesity. Insights gleaned from scientific efforts are broadly communicated to patients, health professionals, and the public through the Institute’s National Digestive Diseases Information Clearinghouse and Weight-control Information Network.
NIDDK established a multi-center Hepatitis B Clinical Research Network in 2008 to collect patient data and samples, conduct a clinical trial of long-term antiviral therapy, and support additional studies of disease development and therapy. NIDDK also modified and expanded the Institute-supported Drug Induced Liver Injury Network in 2008 to enhance studies of genetic and other biological factors that make some people highly susceptible to liver injury from pharmaceuticals, nutritional and herbal supplements, and alternative medicines; and to develop outreach and educational activities for clinicians and the public. In 2006, the NIDDK established a clinical research consortium focused on the causes and potential therapies for gastroparesis (delayed stomach emptying). This consortium was expanded in 2008 to accelerate patient recruitment for the consortium’s patient registry and clinical trials. Building on a scientific workshop held in 2008 on the health and repair of the intestinal tract lining, NIDDK issued a research solicitation to establish an intestinal stem cell research consortium.
Budget Policy: The FY 2010 budget estimate for this program is $462.928 million, an increase of $5.215 million or 1.1 percent above the FY 2009 estimate. In FY 2010, NIDDK will continue major clinical research networks to help understand and treat liver diseases, including hepatitis B, drug-induced liver injury, and nonalcoholic steatohepatitis. NIDDK will also support a Childhood Liver Disease Research and Education Network. Among its obesity-related efforts in FY 2010, NIDDK will support major ongoing observational studies to assess the health risks and benefits of weight-loss surgery in extremely obese adults and adolescents, as well as an ongoing trial evaluating the long-term health effects of weight loss in obese adults with type 2 diabetes (Look AHEAD). NIDDK will also use FY 2010 funds to support Digestive Diseases Research Core Centers, and to sustain a consortium that is conducting cutting-edge genetic research on inflammatory bowel disease (IBD). New research on intestinal stem cells that can benefit a variety of digestive diseases will continue in FY 2010, along with other efforts as part of an overall balanced research portfolio.
Portrait of a Program: Assessing Bariatric Surgery to Combat Obesity
| LABS | Teen-LABS |
|---|
FY2009 Level: | $4.000 Million | $1.580 Million |
FY 2010 Level: | $4.000 Million | $2.381 Million |
Change: | $0.000 Million | + $0.801 Million |
The NIDDK is actively pursuing opportunities to understand both the benefits and risks of gastric bypass surgery, or bariatric surgery, as a treatment for severe obesity. Bariatric surgery procedures reduce stomach size and/or reduce absorption of nutrients. These procedures promote weight loss and especially maintenance of weight loss, and can ameliorate or reverse type 2 diabetes and other obesity-related health problems. However, they also carry significant risks, such as bleeding, infection, blood clots, and malnourishment—a particularly important problem in the face of an increasing number of youth developing severe obesity and its serious health complications, such as sleep apnea and type 2 diabetes, who may choose bariatric surgery as a treatment option.
To learn more about surgery risks and benefits and to identify the kinds of patients most likely to benefit from this type of procedure, NIDDK issued a solicitation to begin a coordinated effort of clinical, epidemiologic, and behavioral research in bariatric surgery. This effort was informed by external input. NIDDK established the multi-center Longitudinal Assessment of Bariatric Surgery (LABS) Consortium in 2003. LABS is rigorously collecting information on a variety of medical, psychosocial, and economic factors both before and after surgery from patients already planning a procedure. The LABS program does not pay for surgery or patient care. The baseline and outcomes information should ultimately provide a comprehensive picture of the risks and benefits of surgery and the characteristics of patients who benefit. Biological samples and data collected from patients will also provide a valuable resource for future study of obesity and its complications. NIDDK is planning to extend LABS for another five years beginning in FY 2009. In 2007, NIDDK launched Teen-LABS to provide a companion observational study of the benefits and risk of these procedures in very severely obese teenagers. Recent advances in research have revealed that bariatric surgery in many cases ameliorates insulin resistance and type 2 diabetes independent of the resulting weight loss. The NIDDK is encouraging research projects on the underlying mechanisms of action in bariatric surgery that produce this salutary health impact, which may in turn lead to new, potentially non-surgical therapeutic approaches to obesity and its complications.
Kidney, Urologic, and Hematologic Diseases: The goals of this program are to increase understanding of diseases and disorders of the kidneys, urinary tract, and blood (hematologic), and to develop and test potential prevention and treatment strategies. Basic, clinical, and translational research, as well as research training, is supported in the areas of chronic kidney disease, diabetic kidney disease, end-stage renal disease (kidney failure), polycystic kidney disease, and many other kidney diseases; urinary incontinence, benign prostatic hyperplasia, interstitial cystitis/painful bladder syndrome, stones, impotence, congenital urologic disorders, and urinary tract infections; and disorders of the blood and blood-forming organs including sickle cell disease, Cooley's anemia, hemochromatosis, and the anemia of inflammation and of chronic disease. Science-based information is communicated to patients, health professionals, and the public through NIDDK’s National Kidney and Urologic Diseases Information Clearinghouse and National Kidney Disease Education Program (NKDEP).
In 2008, NIDDK began a multi-center consortium to study acute kidney injury plus initiated a study to understand why arteriovenous fistulas, which are surgically created for use in patients on hemodialysis, do not always develop to be suitable for dialysis. The NKDEP launched new efforts in 2008 to encourage standardized clinical measures of kidney function and to improve screening and management practices so that health care providers can better identify and treat chronic kidney disease before it progresses to kidney failure. A new Multidisciplinary Approach to the Study of Chronic Pelvic Pain research network was established in 2008 to study interstitial cystitis/painful bladder syndrome and chronic prostatitis. NIDDK also funded a new initiative in 2008 to augment career development training opportunities in urologic research, and an enhanced urology centers program with a stronger emphasis on collaborations, multidisciplinary research, and translational research. In early FY 2008, NIDDK held a strategic planning meeting for hematology research and research training, to solicit broad input on program directions.
Budget Policy: The FY 2010 budget estimate for this program is $447.496 million, an increase of $5.040 million or 1.1 percent above the FY 2009 estimate. In FY 2010, NIDDK will continue support for ongoing major clinical studies of chronic kidney disease in adults and children and fund new research to identify and validate biomarkers and risk assessment tools for patients with this condition. In FY 2010, NIDDK will move forward with treatment trials for polycystic kidney disease (HALT-PKD study) and will continue support for the Consortium for Radiologic Imaging Studies of PKD. Centers focused on kidney, urologic, and hematologic research will receive continued funding, as will research on acute kidney injury and a study of arteriovenous fistulas. Also in FY 2010, NIDDK will support the pilot phase of an initiative to study lower urinary tract symptoms and benign prostatic hyperplasia and continue a recently established research network to study urologic chronic pelvic pain. NIDDK will continue support for the Systolic Blood Pressure Intervention Trial (led by NHLBI) and for other efforts as part of an overall balanced research portfolio.
Portrait of a Program: Chronic Kidney Disease in Children – The CKiD Study
FY 2009 Level: $3.350 Million*
FY 2010 Level: $3.350 Million*
Change: $0.000 Million
NIDDK is committed to combating chronic kidney disease, an increasingly common disease which greatly increases risk for heart disease and stroke and can progress to kidney failure (end-stage renal disease), a condition that requires life-long dialysis or kidney transplantation. In children, chronic kidney disease has additional adverse effects on growth, brain development, cognitive abilities, and behavior—health issues that can affect children’s chances to lead normal adult lives. Moreover, researchers estimate that children on dialysis are thirty times more likely to die than children in the general population. If found early, the disease can be managed so as to delay or prevent kidney failure and cardiovascular disease, but patients with early chronic kidney disease experience no symptoms.
To advance knowledge about the progression of chronic kidney disease and its impacts on children, and to inform the development of future intervention studies to improve patients’ health, NIDDK issued a research solicitation to launch a study of chronic kidney disease in children. This effort was informed by external input. The multi-site Chronic Kidney Disease in Children (CKiD) epidemiologic study, supported by NIDDK with co-funding from other NIH institutes, seeks to identify risk factors for declining kidney function, characterize the impact of chronic kidney disease on children’s neurocognitive development and growth, and examine the effect of chronic kidney disease on risk factors for cardiovascular disease. Begun in 2003, this prospective cohort study has enrolled almost 540 young children and teens with mild to moderately impaired kidney function. Early results have already increased researchers’ knowledge about the health of children with chronic kidney disease. For example, the incidence of low birth-weight in these children is almost three times the national average. CKiD researchers are also making progress toward more accurate ways of calculating children’s kidney function. CKiD was renewed for five years in FY 2008 due to productivity to date and information coming out of the preliminary analyses. With continued support, CKiD can continue to make progress toward improved diagnosis, management, and treatment of chronic kidney disease in children. Biological samples collected during the study will also facilitate genetic and other research in chronic kidney disease.
*Includes $2.900 Million from NIDDK, $0.200 Million from NICHD, and $0.250 Million from NHLBI
Special Statutory Funding Program for Type 1 Diabetes Research: Complementing the efforts of the Diabetes, Endocrinology and Metabolic Diseases program, this special program’s goal is to foster improved treatment, prevention, and cure of type 1 diabetes and its complications through basic, clinical, and translational research framed around six scientific goals: identifying genetic and environmental causes of type 1 diabetes (25.554 million), preventing or reversing the disease (35.635 million), developing cell replacement therapy (48.691 million), preventing or reducing hypoglycemia (12.167 million), preventing or reducing health complications (5.953 million), and attracting new talent and applying new technologies to research (22.000 million) (dollars are FY 2010 estimates). Although focused on type 1 diabetes, aspects of this research will also benefit those with other autoimmune disorders, as well as those with type 2 diabetes. Both type 1 and type 2 diabetes share a basis in impaired function of the insulin producing beta cells of the pancreas along with the same possible complications, such as heart disease, stroke, blindness, kidney failure, nerve damage, and lower limb amputations.
In 2008, NIDDK awarded the first Type 1 Diabetes Pathfinder Awards to support new investigators studying type 1 diabetes or its complications. The Type 1 Diabetes Genetics Consortium completed a genome-wide analysis study aimed at uncovering genetic contributors to this disease. In FY 2007 and FY 2008, the Type 1 Diabetes TrialNet research network made progress in clinical trials of interventions to preserve insulin production in patients newly diagnosed with type 1 diabetes, completing one trial, finishing recruitment for a second trial, and launching a third. NIDDK also recompeted the TrialNet data coordinating center and switched it to a contract mechanism in 2008 to reduce costs and give greater control over data and biosamples.
Budget Policy : The FY 2010 budget estimate for the Special Statutory Funding Program for Type 1 Diabetes Research is $150.000 million, the same as FY 2009. NIDDK administers the program, but because of its trans-HHS nature, the resources are disbursed among multiple NIH Institutes and Centers as well as the CDC. Among ongoing NIDDK-led efforts that will continue with FY 2010 funds are an ambitious study that aims to identify environmental causes of type 1 diabetes in genetically susceptible individuals (TEDDY) (23.304 million), and the Type 1 Diabetes TrialNet research network (27.986 million). The Clinical Islet Transplantation consortium will also be supported in FY 2010 (10.000 million). Research on development of an “artificial pancreas” will be continued in FY 2010 through initiatives funding small business research to develop new therapeutics and monitoring technologies for type 1 diabetes (2.000 million) and clinical research on closed loop technologies. (7.500 million).
Intramural Research: The goal of this program is to conduct basic, translational, and clinical biomedical research related to diabetes and other endocrine and metabolic diseases; digestive diseases, including liver diseases and nutritional disorders; obesity; kidney diseases; and hematologic diseases. Intramural research is conducted in the Institute’s laboratories and clinical facilities in Bethesda, Maryland, as well as in Phoenix, Arizona, where a long-standing research partnership with the Pima Indians in the region has led to important scientific advances in type 2 diabetes and obesity. Research training is also an integral component of the Intramural research program.
In 2008, NIDDK continued these research and training efforts and also helped support the establishment of the NHGRI-led NIH Intramural Center for Genomics and Health Disparities (NICGHD), a new venue for research about the way populations are impacted by diseases, including obesity, diabetes and hypertension.
Budget Policy: The FY 2010 budget estimate for this program is $175.581 million, an increase of $2.595 million or 1.5 percent above the FY 2009 estimate. With FY 2010 funds, the NIDDK Intramural Research Program will continue a broad spectrum of research studies to strengthen understanding of basic biology and disease mechanisms, and evaluate potential therapeutic approaches. For example, in FY 2010 intramural scientists will continue research on obesity in the trans-NIH Metabolic Clinical Research Unit, as well as research relevant to diabetes; digestive diseases, including liver disease; kidney disease; and hematologic disease. The Program will also continue to support research training.
Research Management and Support: RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. RMS functions also encompass strategic planning, coordination, and evaluation of the Institute’s programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public.
Through its RMS activities, NIDDK has continued to fund meritorious basic, clinical, and translational research and research training efforts, and also continued its health information dissemination and education/outreach activities. Additionally, the NIDDK’s strategic planning, evaluation, and other activities have continued; some of these are highlighted above. In another example, NIDDK has provided leadership and support to the National Commission on Digestive Diseases, established by the NIH Director, which recently completed development of a long-range plan for digestive diseases research.
Budget Policy: The FY 2010 budget estimate for RMS is $63.737 million, an increase of $1.096 million or 1.7 percent above the FY 2009 estimate. NIDDK will continue effective research management and support so as to deploy research resources to the most meritorious and promising areas, and to communicate research opportunities and findings to investigators, health professionals, and the public.
Budget Authority by Object | FY 2009 (Estimate) | FY 2010 (PB) | Increase or Decrease |
|---|
| Total compensable workyears: | | | |
| Full-time employment | 618 | 630 | 12 |
| Full-time equivalent of overtime and holiday hours | 3 | 3 | 0 |
| Average ES salary | $159,348 | $162,535 | $3,187 |
| Average GM/GS grade | 12.1 | 12.3 | 0.2 |
| Average GM/GS salary | $91,670 | $93,503 | $1,833 |
| Average salary, grade established by act of | | | |
| July 1, 1944 (42 U.S.C. 207) | $94,169 | $96,052 | $1,883 |
| Average salary of ungraded positions | 125,427 | 127,935 | 2,508 |
OBJECT CLASSES
Personnel Compensation: | FY 2009 (Estimate) | FY 2010 (PB) | Increase or Decrease |
|---|
| 11.1 Full-time permanent | $34,305,000 | $35,412,000 | $1,107,000 |
| 11.3 Other than full-time permanent | 30,236,000 | 31,035,000 | 799,000 |
| 11.5 Other personnel compensation | 1,907,000 | 1,967,000 | 60,000 |
| 11.7 Military personnel | 2,339,000 | 2,409,000 | 70,000 |
| 11.8 Special personnel services payments | 12,964,000 | 13,243,000 | 279,000 |
| Total Personnel Compensation | 81,751,000 | 84,066,000 | 2,315,000 |
| 12.0 Personnel benefits | 18,235,000 | 18,759,000 | 524,000 |
| 12.2 Military personnel benefits | 1,995,000 | 2,057,000 | 62,000 |
| 13.0 Benefits to former personnel | 0 | 0 | 0 |
| Subtotal, Pay Costs | 101,981,000 | 104,882,000 | 2,901,000 |
| 21.0 Travel and transportation of persons | 2,131,000 | 2,118,000 | (13,000) |
| 22.0 Transportation of things | 287,000 | 286,000 | (1,000) |
| 23.1 Rental payments to GSA | 0 | 0 | 0 |
| 23.2 Rental payments to others | 0 | 0 | 0 |
| 23.3 Communications, utilities and miscellaneous charges | 690,000 | 692,000 | 2,000 |
| 24.0 Printing and reproduction | 1,136,000 | 1,125,000 | (11,000) |
| 25.1 Consulting Services | 860,000 | 856,000 | (4,000) |
| 25.2 Other services | 12,160,000 | 12,135,000 | (25,000) |
| 25.3 Purchase of goods and services from government accounts | 151,986,000 | 153,128,000 | 1,142,000 |
| 25.4 Operation and maintenance of facilities | 1,913,000 | 1,922,000 | 9,000 |
| 25.5 Research and development contracts | 107,375,000 | 108,259,000 | 884,000 |
| 25.6 Medical care | 1,129,000 | 1,136,000 | 7,000 |
| 25.7 Operation and maintenance of equipment | 3,651,000 | 3,663,000 | 12,000 |
| 25.8 Subsistence and support of persons | 0 | 0 | 0 |
| 25.0 Subtotal Other Contractual Services | 279,074,000 | 281,099,000 | 2,025,000 |
| 26.0 Supplies and materials | 15,603,000 | 15,602,000 | (1,000) |
| 31.0 Equipment | 10,280,000 | 10,230,000 | (50,000) |
| 32.0 Land and structures | 0 | 0 | 0 |
| 33.0 Investments and loans | 0 | 0 | 0 |
| 41.0 Grants, subsidies and contributions | 1,500,150,000 | 1,515,454,000 | 15,304,000 |
| 42.0 Insurance claims and indemnities | 0 | 0 | 0 |
| 43.0 Interest and dividends | 6,000 | 6,000 | 0 |
| 44.0 Refunds | 0 | 0 | 0 |
| Subtotal, Non-Pay Costs | 1,809,357,000 | 1,826,612,000 | 17,255,000 |
| Total Budget Authority by Object | 1,911,338,000 | 1,931,494,000 | 20,156,000 |
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research
Salaries and Expenses OBJECT CLASSES
Personnel Compensation: | FY 2009 (Estimate) | FY 2010 (PB) | Increase or Decrease |
|---|
| Full-time permanent (11.1) | $34,305,000 | $35,412,000 | $1,107,000 |
| Other than full-time permanent (11.3) | 30,236,000 | 31,035,000 | 799,000 |
| Other personnel compensation (11.5) | 1,907,000 | 1,967,000 | 60,000 |
| Military personnel (11.7) | 2,339,000 | 2,409,000 | 70,000 |
| Special personnel services payments (11.8) | 12,964,000 | 13,243,000 | 279,000 |
| Total Personnel Compensation (11.9) | 81,751,000 | 84,066,000 | 2,315,000 |
| Civilian personnel benefits (12.1) | 18,235,000 | 18,759,000 | 524,000 |
| Military personnel benefits (12.2) | 1,995,000 | 2,057,000 | 62,000 |
| Benefits to former personnel (13.0) | 0 | 0 | 0 |
| Subtotal, Pay Costs | 101,981,000 | 104,882,000 | 2,901,000 |
| Travel (21.0) | 2,131,000 | 2,118,000 | (13,000) |
| Transportation of things (22.0) | 287,000 | 286,000 | (1,000) |
| Rental payments to others (23.2) | 0 | 0 | 0 |
| Communications, utilities and miscellaneous charges (23.3) | 690,000 | 692,000 | 2,000 |
| Printing and reproduction (24.0) | 1,136,000 | 1,125,000 | (11,000) |
| Other Contractual Services: | | | |
| Advisory and assistance services (25.1) | 860,000 | 856,000 | (4,000) |
| Other services (25.2) | 12,160,000 | 12,135,000 | (25,000) |
| Purchases from government accounts (25.3) | 94,727,000 | 95,771,000 | 1,044,000 |
| Operation and maintenance of facilities (25.4) | 1,913,000 | 1,922,000 | 9,000 |
| Operation and maintenance of equipment (25.7) | 3,651,000 | 3,663,000 | 12,000 |
| Subsistence and support of persons (25.8) | 0 | 0 | 0 |
| Subtotal Other Contractual Services | 113,311,000 | 114,347,000 | 1,036,000 |
| Supplies and materials (26.0) | 15,591,000 | 15,590,000 | (1,000) |
| Subtotal, Non-Pay Costs | 133,146,000 | 134,158,000 | 1,012,000 |
| Total, Administrative Costs | 235,127,000 | 239,040,000 | 3,913,000 |
Authorizing Legislation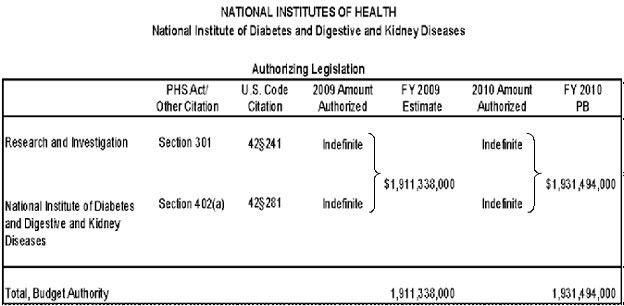 Appropriations History
Appropriations History | Fiscal Year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation |
|---|
| Fiscal Year 2001 | 1,186,266,0002 | 1,315,530,000 | 1,318,106,000 | 1,470,385,000 |
| Rescission 2001 | | | | (429,000) |
| Fiscal Year 2002 | 1,457,915,000 | 1,446,705,000 | 1,501,476,000 | 1,563,833,000 |
| Rescission 2002 | | | | (453,000) |
| Fiscal Year 2003 | 1,706,292,000 | 1,731,754,000 | 1,731,754,000 | 1,733,347,000 |
| Rescission 2003 | | | | (10,617,000) |
| Fiscal Year 2004 | 1,820,000,0003 | 1,820,007,000 | 1,833,007,000 | 1,821,240,000 |
| Rescission 2004 | | | | (10,654,000) |
| Fiscal Year 2005 | 1,877,696,0003 | 1,876,196,000 | 1,889,100,000 | 1,863,584,000 |
| Rescission 2005 | | | | (14,112,000) |
| Fiscal Year 2006 | 1,872,146,0003 | 1,872,146,000 | 1,917,919,000 | 1,854,925,000 |
| Rescission 2006 | | | | (17,221,000) |
| Fiscal Year 2007 | 1,844,298,0003 | 1,844,298,000 | 1,857,753,000 | 1,855,868,000 |
| Rescission 2007 | | | | 0 |
| Fiscal Year 2008 | 1,858,045,0003 | 1,881,893,000 | 1,897,784,000 | 1,864,945,000 |
| Rescission 2008 | | | | (30,331,000) |
| Supplemental | | | | 9,077,000 |
| Fiscal Year 2009 | 1,858,487,0003 | 1,767,071,000 | 1,755,881,000 | 1,911,338,000 |
| Rescission 2009 | | | | 0 |
| Fiscal Year 2010 | 1,931,494,0003 | | | |
1 Reflects enacted supplementals, rescissions, and reappropriations.
2 Excludes funds for HIV/AIDS research activities consolidated in the NIH Office of AIDS Research
3 Includes Type 1 Diabetes Special Statutory Authority Funds
Details of Full-Time Equivalent Employment (FTEs) | OFFICE/DIVISION | FY 2008 (Actual) | FY 2009 (Estimate) | FY 2010 (PB) |
|---|
| Office of the Director | 87 | 83 | 85 |
| Division of Diabetes, Endocrinology, and Metabolic Diseases | 26 | 26 | 26 |
| Division of Digestive Diseases and Nutrition | 21 | 21 | 21 |
| Division of Kidney, Urologic, and Hematologic Diseases | 20 | 20 | 20 |
| Division of Nutrition Research Coordination | 9 | 9 | 9 |
| Division of Extramural Activities | 82 | 76 | 78 |
| Division of Intramural Research | 401 | 383 | 391 |
| Total | 646 | 618 | 630 |
| Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research |
FTEs supported by funds from the Cooperative Research and
Development Agreements | (4) | (4) | (4) |
| FISCAL YEAR | Average GM/GS Grade |
|---|
| 2006 | 11.5 |
| 2007 | 11.6 |
| 2008 | 11.8 |
| 2009 | 12.1 |
| 2010 | 12.3 |
Details of Positions | GRADE | FY 2008 (Actual) | FY 2009 (Estimate) | FY 2010 (PB) |
|---|
| Total ES Positions | 1 | 1 | 1 |
| Total, ES Salary | 152,079 | 159,348 | 162,535 |
| GM/GS-15 | 40 | 35 | 35 |
| GM/GS-14 | 61 | 58 | 62 |
| GM/GS-13 | 70 | 74 | 76 |
| GS-12 | 61 | 64 | 68 |
| GS-11 | 43 | 46 | 46 |
| GS-10 | 0 | 0 | 0 |
| GS-9 | 30 | 24 | 24 |
| GS-8 | 23 | 18 | 18 |
| GS-7 | 21 | 15 | 15 |
| GS-6 | 2 | 0 | 0 |
| GS-5 | 4 | 2 | 2 |
| GS-4 | 2 | 2 | 2 |
| GS-3 | 1 | 0 | 0 |
| GS-2 | 0 | 0 | 0 |
| GS-1 | 0 | 0 | 0 |
| Subtotal | 358 | 338 | 348 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): Assistant Surgeon General | 1 | 1 | 1 |
| Director Grade | 13 | 13 | 13 |
| Senior Grade | 3 | 3 | 3 |
| Full Grade | 4 | 4 | 4 |
| Senior Assistant Grade | 1 | 2 | 2 |
| Assistant Grade | 1 | 0 | 0 |
| Subtotal | 23 | 23 | 23 |
| Ungraded | 266 | 258 | 260 |
| Total permanent positions | 379 | 363 | 370 |
| Total positions, end of year | 648 | 620 | 632 |
| Total full-time equivalent (FTE) employment, end of year | 646 | 618 | 630 |
| Average ES salary | 152,079 | 159,348 | 162,535 |
| Average GM/GS grade | 11.8 | 12.1 | 12.3 |
| Average GM/GS salary | 87,488 | 91,670 | 93,503 |
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research
[1] Centers for Disease Control and Prevention. National Diabetes Fact Sheet, United States, 2007.
[2] NIDDK, NIH/DHHS. Kidney and urologic diseases statistics (http://kidney.niddk.nih.gov/statistics/) 2008;. USRDS 2008 Annual Data Report, NIH, NIDDK.
[3] NIDDK, NIH/DHHS. Digestive diseases statistics, 2005 (http://digestive.niddk.nih.gov/statistics).
[4] NIDDK, NIH/DHHS. Statistics related to overweight and obesity, 2007 (http://www.win.niddk.nih.gov/
statistics/index.htm); JAMA 299:2401, 2008; National Center for Health Statistics, Data Brief no 1, 2007.
Page last updated: May 07, 2009