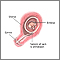
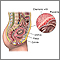
Chorionic villus sampling
Chorionic villus sampling (CVS) is a test done on some pregnant women. It is the removal of a small piece of tissue from the placenta from the womb during early pregnancy. It is done to screen the baby for genetic problems.
How the Test is Performed
There are two ways to do this test:
- Through the cervix (transcervical): a thin plastic tube is placed through the vagina and cervix to reach the placenta
- Through your belly (transabdominal): a needle is place through the belly and womb and into the placenta.
Both methods are equally safe when done by an experienced health care provider. However, the chance of miscarriage is higher when the test is done through the cervix.
An ultrasound of your belly area (abdominal ultrasound ) is done first to determine the safest method to use. The ultrasound helps your doctor determine the position of the womb, the size of the gestational sac, and the position of the placenta.
Before the test is done, your vulva, vagina, cervix, and abdomen will be cleaned with an germ-killing solution (antiseptic). Ultrasound is used to guide your doctor to the correct area, regardless of which method is used.
The test takes about 5 minutes. The sample is sent to a lab for examination. Test results take about 2 weeks.
How to Prepare for the Test
Your doctor or nurse will explain the procedure, its risks, and alternative procedures such as amniocentesis.
Genetic counseling is recommended prior to the procedure. This will allow you to make an unhurried, informed decision regarding options for prenatal diagnosis.
Before the test, you may be asked to drink fluids and avoid urinating so your bladder is full for the test. A full bladder helps your doctor better see the womb area.
How the Test Will Feel
The ultrasound doesn't hurt.
The antiseptic cleansing solution will feel cold at first and may irritate your skin if not washed off after the procedure.
Some women say the vaginal approach feels like a Pap smear with some discomfort and a feeling of pressure. There may be a small amount of vaginal bleeding following the procedure.
Why the Test is Performed
Your doctor may recommend this test to screen your baby for certain genetic disorders.
It can be done sooner in pregnancy than amniocentesis, usually about 10 to 12 weeks of pregnancy.
Chorionic villus sampling does not detect neural tube defects. If neural tube defects or Rh incompatibility are a concern, an amniocentesis will be performed.
Normal Results
A normal result means there are no signs of any genetic defects in the developing baby. However the test could miss some genetic defects.
Note: Normal value ranges may vary slightly among different laboratories. Talk to your doctor about the meaning of your specific test results.
What Abnormal Results Mean
This test can help detect more than 200 disorders. Abnormal results may be due to a number of different genetic conditions, including:
- Down syndrome
- Hemoglobinopathies
- Tay-Sachs disease
Risks
The risks of CVS are only slightly higher than those of an amniocentesis.
Possible complications include:
- Bleeding
- Infection
- Miscarriage
- Rh incompatibility in the mother
- Rupture of membranes
Signs of complications include:
- Excessive bleeding
- Excessive vaginal discharge
- Fever
Immediately tell your doctor or nurse about any complications or concerns.
This test was previously thought to cause arm or leg problems in the developing baby. However, when it is done after 9 weeks of pregnancy, the risk appears to be very low (6 per 10,000). This is no more frequent than in pregnancies without such testing.
Considerations
If your blood is Rh negative, you may receive RhoGAM to prevent Rh incompatibility.
You will receive a follow-up ultrasound 2 to 4 days after the procedure to make sure the pregnancy is proceeding normally.
References
Simpson JL, Richards DS, Otano L Driscoll DA. Prenatal genetic diagnosis. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics: Normal and Problem Pregnancies. 6th ed. Philadelphia, Pa: Saunders Elsevier; 2012:chap11.
American College of Obstetricians and Gynecologists (ACOG). Invasive prenatal testing for aneuploidy. Washington (DC): American College of Obstetricians and Gynecologists (ACOG); 2007 Dec. 9 p.
Update Date: 5/31/2012
Updated by: Linda J. Vorvick, MD, Medical Director and Director of Didactic Curriculum, MEDEX Northwest Division of Physician Assistant Studies, Department of Family Medicine, UW Medicine, School of Medicine, University of Washington. Susan Storck, MD, FACOG, Chief, Eastside Department of Obstetrics and Gynecology, Group Health Cooperative of Puget Sound, Bellevue, Washington; Clinical Teaching Faculty, Department of Obstetrics and Gynecology, University of Washington School of Medicine. Also reviewed by David Zieve, MD, MHA, Medical Director, A.D.A.M. Health Solutions, Ebix, Inc.
MedlinePlus Topics
Images
Read More

A.D.A.M., Inc. is accredited by URAC, also known as the American Accreditation HealthCare Commission (www.urac.org). URAC's accreditation program is an independent audit to verify that A.D.A.M. follows rigorous standards of quality and accountability. A.D.A.M. is among the first to achieve this important distinction for online health information and services. Learn more about A.D.A.M.'s editorial policy, editorial process and privacy policy. A.D.A.M. is also a founding member of Hi-Ethics and subscribes to the principles of the Health on the Net Foundation (www.hon.ch).
The information provided herein should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. A licensed physician should be consulted for diagnosis and treatment of any and all medical conditions. Call 911 for all medical emergencies. Links to other sites are provided for information only -- they do not constitute endorsements of those other sites. Copyright 1997-2012, A.D.A.M., Inc. Duplication for commercial use must be authorized in writing by ADAM Health Solutions.