FY2012 Budget
DEPARTMENT OF HEALTH AND HUMAN SERVICES
NATIONAL INSTITUTES OF HEALTH
National Heart, Lung, and Blood Institute(NHLBI)
- Organization chart
- Appropriation language
- Amounts available for obligation
- Budget mechanism table
- Major changes in budget request
- Summary of changes
- Budget graphs
- Budget Authority by Activity
- Authorizing legislation
- Appropriations history
- Justification of Budget Request
- Budget Authority by Object Class
- Salaries and expenses
- Detail of full-time equivalent employment (FTE)
- Detail of positions
- New positions requested
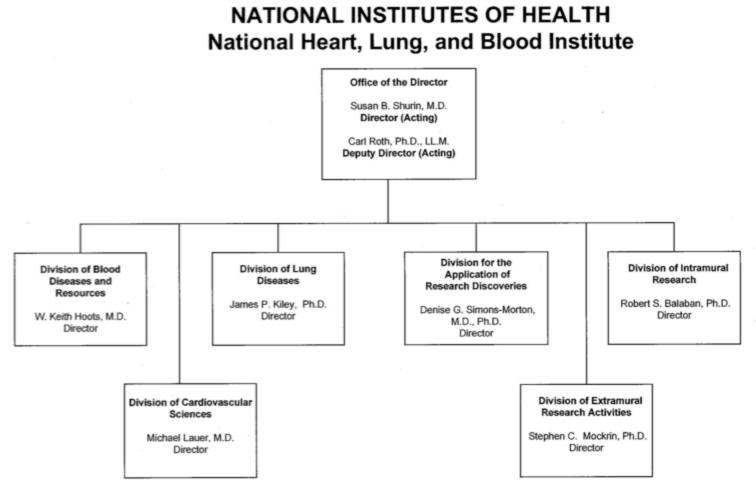
Organization chart
For carrying out section 301 and title IV of the Public Health Services Act with respect to cardiovascular, lung, and blood diseases and blood products $3,147,992,000.
Amounts Avaliable for Obligation (1)
(Dollars in Thousands)
Source of Funding |
FY 2010 Actual |
FY 2011 CR |
FY 2012 PB |
|---|---|---|---|
| Appropriation | $3,096,916 | $3,096,916 | $3,147,992 |
| Type 1 Diabetes | 0 | 0 | 0 |
| Rescission | 0 | 0 | 0 |
| Supplemental | 0 | 0 | 0 |
| Subtotal, adjusted appropriation |
$3,096,916 | $3,096,916 |
$3,147,992 |
| Real transfer under Director's one-percent transfer authority (GEI) | -$2,845 | 0 | 0 |
| Real transfer under Secretary's one-percent transfer authority | -$463 | 0 | 0 |
| Comparative Transfers to NLM for NCBI and Public Access | -$1,182 | -$2,634 | 0 |
| Comparative transfer under Director's one-percent transfer authority (GEI) | $2,845 | 0 | 0 |
| Comparative transfer under Secretary's one-percent transfer authority | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | $3,095,271 | $3,094,282 | $3,147,992 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligatied balance, end of year | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | $3,095,271 | $3,094,282 | $3,147,992 |
| Unobligated balance lapsing | -$107 | 0 | 0 |
| Total obligation | $3,095,164 | $3,094,282 | $3,147,992 |
(1)Excludes the following amounts for reimbursable activities carried out by this account:
FY 2010 - $50,029 FY 2011 - $25,000 FY 2012 - $25,000
Budget Mechanism-Total
(dollars in thousands)
MECHANISM |
FY 2010 Actual |
FY 2011 CR |
FY 2012 PB |
Change vs. FY 2010 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Research Grants: | No. | Amount | No. | Amount | No. | Amount | No. | Amount | ||||
| Research Projects: | ||||||||||||
| Noncompeting | 2,959 | $1,554,173 | 2,948 | $1,585,000 | 2,825 | $1,598,748 | (134) | $44,575 | ||||
| Administrative supplements | (119) | 7,500 | (119) | 7,700 | (119) | 7,000 | 0 | (500) | ||||
| Competing: | ||||||||||||
| Renewal | 262 | 153,880 | 237 | 141,279 | 251 | 151,095 | (11) | (2,785) | ||||
| New | 640 | 313,928 | 579 | 288,222 | 613 | 308,247 | (27) | (5,681) | ||||
| Supplements | 1 | 247 | 0 | 0 | 0 | 0 | (1) | (247) | ||||
| Subtotal, Competing | 903 | $468,055 | 816 | $429,501 | 864 | $459,342 | (39) | ($8,713) | ||||
| Subtotal, RPGs | 3,862 | $2,029,728 | 3,764 | $2,022,201 | 3,689 | $2,065,090 | (173) | $35,362 | ||||
| SBIR/STTR | 165 | $76,400 | 165 | $71,000 | 165 | $71,000 | 0 | ($5,400) | ||||
| Research Project Grants | 4,027 | $2,106,128 | 3,929 | $2,093,201 | 3,854 | $2,136,090 | (173) | $29,962 | ||||
| Research Centers: | ||||||||||||
| Specialized/comprehensive | 39 | $71,650 | 62 | $59,920 | 53 | $60,000 | 14 | ($11,650) | ||||
| Clinical research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Biotechnology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Comparative medicine | 0 | 855 | 0 | 455 | 0 | 455 | 0 | (400) | ||||
| Research Centers in Minority Institutions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Research Centers | 39 | $72,505 | 62 | $60,375 | 53 | $60,455 | 14 | ($12,050) | ||||
| Other Research: | ||||||||||||
| Research careers | 591 | $81,701 | 591 | $81,701 | 591 | $82,518 | 0 | $817 | ||||
| Cancer education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Cooperative clinical research | 11 | 18,650 | 44 | 43,000 | 48 | 47,000 | 37 | 28,350 | ||||
| Biomedical research support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Minority biomedical research support | 11 | 2,608 | 14 | 3,000 | 16 | 3,200 | 5 | 592 | ||||
| Other | 120 | 26,300 | 120 | 26,000 | 120 | 26,000 | 0 | (300) | ||||
| Other Research | 733 | $129,259 | 769 | $153,701 | 775 | $158,718 | 42 | $29,459 | ||||
| Total Research Grants | 4,799 | $2,307,892 | 4,760 | $2,307,277 | 4,682 | $2,355,263 | (117) | $47,371 | ||||
| Research Training | FTTPs | FTTPs | FTTPs | |||||||||
| Individual Awards | 275 | $11,283 | 271 | $11,283 | 271 | $11,734 | (4) | $451 | ||||
| Institutional Awards | 1,777 | 86,711 | 1,768 | 86,711 | 1,768 | 89,313 | (9) | 2,602 | ||||
| Total Research Training | 2,052 | $97,994 | 2,039 | $97,994 | 2,039 | $101,047 | (13) | $3,053 | ||||
| Research & development contracts | 170 | $384,437 | 170 | $376,921 | 170 | $376,921 | 0 | ($7,516) | ||||
| SBIR/STTR | (15) | ($2,538) | (20) | ($7,500) | (20) | ($8,000) | (5) | $5,462 | ||||
| FTEs | FTEs | FTEs | FTEs | |||||||||
| Intramural Research | 455 | $185,304 | 456 | $189,455 | 456 | $190,900 | 1 | $5,596 | ||||
| Research management and support | 421 | 119,644 | 422 | 122,635 | 422 | 123,861 | 1 | 4,217 | ||||
| Construction | 0 | 0 | 0 | 0 | ||||||||
| Buildings and Facilities | 0 | 0 | 0 | 0 | ||||||||
| Total, NHLBI | 876 | $3,095,271 | 878 | $3,094,282 | 878 | $3,147,992 | 2 | $52,721 | ||||
1/ All items in italics are "non-adds". Items in parentheses are subtractions.
Major Changes in the Fiscal Year 2012 Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2012 budget request for NHLBI, which is $52.721 million more than the FY 2010 Actual, for a total of $3,147.992 million.
Research Project Grants (RPGs; +$29.962 million; total $2,136.090 million): NHLBI will continue to fund of competing RPGs—864 awards in FY 2012, a decrease of 39 from FY 2010. About 2,825 noncompeting RPG awards, totaling $1,598.748 million will be made in FY 2012.
Clinical Hematology Research Career Developmental Program (+$2.700 million; total $13.500 million): Develop and maintain multidisciplinary career development programs in clinical hematology research to equip new academic researchers with the knowledge and skills to address complex problems in blood diseases, transfusion medicine and cellular therapies.
Pediatric Hydroxyurea Phase II Clinical Trial (Baby Hug) (+$1.904 million; total $11.045 million): The objective of this RFP is to investigate the beneficial and adverse effects of early intervention with HU on very young children (ages 9-17 months) with sickle cell disease (SCD) through the first decade of life. In addition, to evaluate organ function, growth and psychosocial development, and predictive value of biomarkers in this cohort of children.
Pulmonary Vascular-Right Ventricular Axis Research Program (+ $4.050 million; total $20.250 million): The objective of this RFA is to identify novel cellular and molecular mechanisms of right ventricular (RV) function and dysfunction and define human RV disease in the setting of lung vascular pathology through multidisciplinary, collaborative research.
Consortium of Lung Repair and Regeneration (+$4.900 million; total $26.300 million): The objective of this RFA is to encourage multidisciplinary, innovative, and collaborative research to identify molecular and cellular mechanisms of lung repair and regeneration, and support development of pioneering tools, reagents, and model systems to enhance such investigations.
Cardiovascular Cell Therapy Research Network (CCTRN) (+$9.000 million; total $63.000 million): This initiative was originally released in FY 2007. The renewal proposes to extend the CCTRN to a second project period for an additional 7 years to continue to accelerate the field of cell-based therapies for cardiovascular diseases.
Basic Research in Calcific Aortic Valve Disease (+$4.000 million; total $20.000 million): This initiative will stimulate studies on calcific aortic valve disease (CAVD), encourage development of new animal models and research techniques, and recruit investigators from related fields, such as mineralization and bone physiology, extracellular matrix physiology, cell signaling, immunology, and molecular imaging.
Summary of Changes
(Dollars in Thousands)
| FY 2010 Actual | $3,095,271 |
|---|---|
| FY 2012 Estimate | 3,147,992 |
| Net change | $52,721 |
| 2012 Estimate | Change
from FY 2010 |
|||
|---|---|---|---|---|
CHANGES |
FTEs |
Budget Authority |
FTEs |
Budget Authority |
| A. Built-in: | ||||
| 1. Intramural research: | ||||
|
a. Annualization of January 2010 pay increase |
$72,019 | $427 | ||
| b. January FY 2012 pay increase | 72,019 | 0 | ||
| c. One less day of pay (n/a for 2011) | 72,019 | (279) | ||
|
d. Payment for centrally furnished services |
28,810 | 285 | ||
|
e. Increased cost of laboratory supplies,
materials, and other expenses |
90,071 | 883 | ||
| Subtotal | $1,316 | |||
| 2. Research management and support: | ||||
|
a. Annualization of January 2010 pay increase |
$62,236 | $368 | ||
| b. January FY 2012 pay increase | 62,236 | $368 | ||
| c. One less day of pay (n/a for 2011) | 62,236 | (241) | ||
| d. Payment for centrally furnished services | 20,015 | 198 | ||
|
e. Increased cost of laboratory supplies, materials, and other expenses |
41,610 | 400 | ||
| Subtotal | $725 | |||
| Subtotal, Built-in | $2,041 | |||
| 2012 Estimate | Change from FY 2010 | |||
|---|---|---|---|---|
| CHANGES | No. | Amount | No. | Amount |
| B. Program: | ||||
| 1. Research project grants: | ||||
| a. Noncompeting | 2,825 | $1,605,748 | (134) | $44,075 |
| b. Competing | 864 | 459,342 | (39) | (8,713) |
| c. SBIR/STTR | 165 | 71,000 | 0 | (5,400) |
| Total | 3,854 | $2,136,090 | (173) | $29,962 |
| 2. Research centers | 53 | $60,455 | 14 | ($12,050) |
| 3. Other research | 775 | 158,718 | 42 | 29,459 |
| 4. Research training | 2,039 | 101,047 | (13) | 3,053 |
| 5. Research and development contracts | 170 | 376,921 | 0 | (7,516) |
| Subtotal, extramural | $697,141 | $42,908 | ||
| FTEs | FTEs | |||
| 6. Intramural research | 456 | $190,900 | 1 | $4,280 |
| 7. Research management and support | 422 | 123,861 | 1 | 3,492 |
| 8. Construction | 0 | 0 | ||
| 9. Buildings and Facilities | 0 | 0 | ||
| Subtotal, program | 878 | $3,147,992 | 2 | $50,680 |
| Total changes | $3,147,992 | $52,721 | ||
Fiscal Year 2011 Budget Graphs
History of budget Authority and FTEs
Distribution by Mechanism
Change by Selected Mechanism
Budget Authority by Activity
(dollars in thousands)
| FY 2010 Actual |
FY 2011 CR |
FY 2012 PB |
Change vs. FY 2010 |
|||||
|---|---|---|---|---|---|---|---|---|
| Extramural Research | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount |
| Detail: | ||||||||
| Heart and Vascular Diseases | $1,771,213 | $1,766,220 | $1,798,621 | $27,408 | ||||
| Lung Diseases | $629,938 | $627,998 | $639,519 | $9,581 | ||||
| Blood Diseases and Resources | $389,172 | $387,974 | $395,091 | $5,919 | ||||
| Subtotal, Extramural | $2,790,323 | $2,782,192 | $2,833,231 | $42,908 | ||||
| Intramural research | 455 | $185,304 | 456 | $189,455 | 456 | $190,900 | 1 | $1,445 |
| Res. management & support | 421 | $119,644 | 422 | $122,635 | 422 | $123,861 | 1 | $1,226 |
| TOTAL | 876 | $3,095,271 | 878 | $3,094,282 | 878 | $3,147,992 | 2 | $45,579 |
1 Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research
2 Includes Real Transfers and Comparable Adjustments as detailed in the "Amounts Available for Obligation" table.
Appropriations History
| Fiscal Year |
Budget Estimate to Congress |
House Allowance |
Senate Allowance |
Appropriation |
|---|---|---|---|---|
| 2003 | $2,778,728,000 | $2,791,411,000 | $2,820,011,000 | $2,812,011,000 |
| Rescission | ($18,278,000) | |||
| 2004 | $2,867,995,000 | $2,867,995,00 | $2,897,595,000 | $2,897,145,000 |
| Rescission | ($18,454,000) | |||
| 2005 | $2,963,953,000 | $2,963,953,000 | $2,985,900,000 | $2,965,453,000 |
| Rescission | ($24,252,000) | |||
| 2006 | 2,951,270,000 | 2,951,270,000 | 3,023,381,000 | 2,951,270,000 |
| Rescission |
(29,513,000) |
|||
| 2007 | 2,918,808,000 | 2,901,012,000 | 2,924,299,000 | 2,918,808,000 |
| Rescission |
0 |
|||
| 2008 | 2,894,341,000 | 2,965,775,000 | 2,992,197,000 | 2,974,900,000 |
| Rescission | (51,972,000) | |||
| Supplemental |
15,542,000 |
|||
| 2009 | 2,924,942,000 | 3,025,500,000 | 3,006,344,000 | 3,015,689,000 |
| Rescission | 0 | |||
| 2010 | 3,050,356,000 | 3,123,403,000 |
3,066,827,000 |
3,096,916,000 |
| Rescission |
0 |
|||
| 2011 | 3,187,516,000 | $3,182,524,000 |
||
| Rescission | ||||
| 2012 | $3,147,992,000 |
Justification of Budget Request
Authorizing Legislation: Section 301 and title IV of the Public Health Service Act, as amended.
| FY2010 Actual | FY 2011 Continuing Resolution |
FY 2012 Budget Request |
FY 2012 +/- FY 2010 |
|
|---|---|---|---|---|
BA |
$3,095,271,000 | $3,094,282,000 | $3,147,992,000 | +52,721,000 |
FTE |
876 | 878 | 878 | +2 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
DIRECTOR'S OVERVIEW
The NHLBI provides leadership for a research and education program to promote prevention and treatment of heart, blood vessel, lung, and blood diseases. Guided by Shaping the Future of Research: A Strategic Plan, the NHLBI supports a robust, collaborative research enterprise, in partnership with private and public organizations, to address the scientific and educational needs of the nation.
The NHLBI has long maintained a balanced portfolio of basic and clinical research activities and, in recent years, has increased its investment in translational approaches to bridge the gap between fundamental discoveries and clinical practice. This emphasis is very much in line with broad based NIH efforts (e.g., Therapeutics for Rare and Neglected Diseases [TRND] and Rapid Access to Interventional Development [RAID]) in translation. Strategies being used by the NHLBI to further this important work include:
- Clinical research networks—infrastructures to enable the rapid development and conduct of multiple clinical protocols for assessment of promising diagnostic and therapeutic strategies for selected disease areas within the mandate of the Institute.
- Production Assistance for Cell Therapy (PACT)—provision of cell-manufacturing facilities and technical expertise essential for advancing cellular therapy research in the areas of regeneration of damaged/diseased tissues, organs, and biologic systems as treatments for serious diseases that do not have effective therapies.
- Bench-to-Bassinet Program—a pediatric cardiovascular program consisting of three interacting entities: the Cardiovascular Development Consortium, to investigate the transcriptional regulatory networks that govern cardiac development, using complementary animal models; the Pediatric Cardiac Genomics Consortium, to speed discovery of causative genes and evaluate the effects of genetic variation on short- and long-term outcomes in patients with congenital heart disease; and the Pediatric Heart Network, to conduct clinical trials.
- Cardiac Translational Research Implementation Program (C-TRIP)—a two-stage program to accelerate the translation of promising therapeutic interventions (derived from fundamental research discoveries) for the treatment and prevention of heart failure or arrhythmias. Twelve planning and development awards were made in FY 2010, and several clinical trials of safety and efficacy that result from this initial effort will be awarded in FY 2012.
- Grand Opportunities Translational Research Implementation Program (GO TRIP)—a program modeled on the C-TRIP, but applicable to all diseases and conditions within the mandate of the NHLBI. GO TRIP’s initial stage was funded via the American Recovery and Reinvestment Act (ARRA), and the Institute is committed to supporting successful projects that result from this investment.
- Phase II Clinical Trials of Novel Treatments for Lung Diseases—support for proof-of-concept testing of interventions that have the potential to revolutionize clinical management of a lung disease or sleep disorder.
- Science Moving TowArds Research Translation and Therapy (SMARTT)—provision of resources for preclinical- and clinical-grade production and testing of new molecular entities for the treatment of heart, lung, and blood diseases.
- Centers for Advanced Diagnostics and Experimental Therapeutics in Lung Diseases—development of diagnostics and/or therapeutics for lung diseases and sleep-disordered breathing that will emphasize structured interactions, collaborations, and data-sharing among participants.
- NHLBI Clinical Trial Pilot Studies—a program to enable more competitive investigator-initiated clinical trial grant applications and more robust and successful clinical trials that evaluate interventions for the treatment or prevention of heart, lung, blood, or sleep disorders.
- Planning Grants for Pivotal Clinical Trials in Hemoglobinopathies—support of activities necessary to assess feasibility and to develop pilot studies required for the design of full-scale clinical trials.
The Institute is also deeply committed to ensuring that the findings of its research programs are rapidly incorporated into public health programs and into the everyday practice of therapeutic and preventive medicine. Public outreach in asthma, women’s heart health, and COPD continues to yield positive results. The NHLBI is developing updated evidence-based guidelines for hypertension, blood cholesterol, and obesity and preparing integrated guidelines to aid practitioners in managing patients with multiple conditions. New guidelines are being developed for pediatric cardiovascular disease prevention and for sickle cell disease and will be widely disseminated.
Over the past several years the Institute has placed strong emphasis on use of cutting-edge approaches to explore genetic influences on heart, lung, and blood diseases. All NHLBI-supported genotyping efforts have been made available for further research by qualified investigators, thus maximizing the scientific return possible from these valuable data. Beginning with genotyping the participants of the Framingham Heart Study, the NHLBI has made a substantial investment in supporting genotyping of its well-phenotyped cohorts. For example, the SNP Typing for Association with Multiple Phenotypes from Existing Epidemiological Data (STAMPEED) program was developed to support studies to identify genetic variants related to heart, lung, and blood disorders and their risk factors using genome-wide association studies. The NHLBI initiated the Candidate-gene Association Resource (CARe) to create a shared genotype/phenotype resource comprising nine NHLBI-funded cohort studies; it also is required to be made available for research by other investigators. More recently, six ARRA-funded signature projects were supported for exome sequencing of additional well-phenotyped NHLBI population cohorts. These one-time investments in a shared genetic association database have already yielded significant findings that are exploited to identify disease mechanisms and therapeutic targets; the ongoing availability of these data is expected to yield unprecedented scientific and medical advances as investigators take advantage of this rich source of information.
The NHLBI also intends to continue its interest in supporting efforts to develop the therapeutic and curative potential of stem cells. Four projects were funded via ARRA to address important gaps in knowledge of induced pluripotent stem cells, including the extent to which they are equivalent to embryonic stem cells and whether they can be used for clinical research in the future. Because many critical questions remain about the utility in regenerative medicine of induced pluripotent stem cells or even embryonic stem cells, NHLBI fully expects to continue its investment in this critically important area.
Overall Budget Policy: The FY 2012 request for NHLBI is $3,147.992 million, an increase of $52.721 million or +1.7 percent over the FY 2010 actual. NHLBI will continue to support early stage investigators and to maintain a balance between solicitations issued to the extramural community and funding made available to support investigator-initiated projects. In FY 2012, NHLBI is providing a 1 percent inflationary increase for non-competing and 2.4 percent for competing grants. The Institute also seeks priority to support highly meritorious new research projects and ongoing initiatives. In addition, NHLBI will provide an increase of four percent for stipends levels under the Ruth L. Kirschstein National Research Service Award training program to continue efforts to attain the stipend levels recommended by the National Academy of Sciences. This will build on the two percent increase in stipend levels for FY 2011. Stipend levels were largely flat for several years, and the requested increase will help to sustain the development of a highly qualified biomedical research workforce. Intramural Research and Research Management and Support will receive an increase to help cover support of ongoing research and modest expansion of new programs. Funds are included in R&D contracts to reflect NHLBI’s share of NIH-wide funding required to support several trans-NIH initiatives, such as the Therapies for Rare and Neglected Diseases program, the Basic Behavioral and Social Sciences Opportunity Network (OppNet), and support for a new synchrotron at the Brookhaven National Laboratory. For example, each IC that will benefit from the new synchrotron will provide funding to total NIH’s commitment to support this new technology--$10 million.
Program Descriptions and Accomplishments
Heart and Vascular Diseases
This program supports research on the causes, diagnosis, treatment, and prevention of heart and vascular diseases. Research areas include atherothrombosis, coronary artery disease, myocardial infarction and ischemia, heart failure, arrhythmia, sudden cardiac death, adult and pediatric congenital heart disease, cardiovascular complications of diabetes and obesity, and hypertension. The program’s efforts encompass basic, translational, clinical, epidemiological, behavioral, nutritional, comparative-effectiveness, international, and health services research disciplines. In FY 2010, NHLBI initiated collaborative research projects to develop an integrated understanding of cardiomyocyte mitochondria and its contributions to myocardial adaptations and heart disease progression, renewed support for the Resuscitation Outcomes Consortium, and established Cardiovascular Outcomes Research Centers. In FY 2011, the Institute will fund a renewal of its Pediatric Heart Network and a follow-up study of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) clinical trial.
Budget Policy: The FY 2012 budget estimate for the Heart and Vascular Diseases program is $1,798.621 million, an increase of $27.408 million or 1.55% over the FY 2010 actual. During FY 2012 NHLBI plans to continue support of genome-wide studies. The program plans for FY 2012 include support for an initiative to conduct large scale, high density, high throughput 3rd generation genome-wide genotyping to characterize genetic variation among member of the Hispanic Community Health Study/Study of Latinos. This initiative will create the infrastructure necessary to identify disease-related genes, explore context-dependent effects, and leverage the phenotypic resources of the largest existing Hispanic study cohort.
Portrait of a Program: Cardiac Translational Research Implementation Program (C-TRIP) FY 2010 Level: $ 8.665 million The C-TRIP is a two-phase initiative to accelerate translation of promising new therapeutic interventions derived from fundamental research discoveries for the treatment and prevention of heart failure or arrhythmias. It supports the planning and execution of well-designed clinical trials to demonstrate safety and efficacy. In the initial phase begun during FY 2010, twelve exploratory planning grants were awarded for two-year project periods to enable study development and trial planning. During FY 2012, the awardees will be eligible to compete for five years of funding to perform human-subject trials of new therapeutic interventions with a primary goal of providing evidence of efficacy and safety. It is expected that up to four awards will be made for interventional trials that show promise of completing all milestones established during the initial phase and evidence of readiness for implementation upon award. |
Lung Diseases
This program supports research on the causes, diagnosis, treatment, and prevention of lung diseases and sleep disorders. Research areas include asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, critical care and acute lung injury, developmental biology and pediatric pulmonary diseases, immunology and fibrosis, lung cell and vascular biology, and pulmonary complications of AIDS and tuberculosis. The National Center on Sleep Disorders Research is administered within the Lung Diseases program. In FY 2010, the NHLBI funded the Prematurity and Respiratory Outcomes Program (PROP) to investigate molecular mechanisms that contribute to respiratory disease risk of the premature newborn and supported planning grants for clinical trials of lung transplantation. In FY 2011, the NHLBI will initiate research to explore common pathogenetic mechanisms of lung cancer and COPD and renew the Severe Asthma Research Program.
Budget Policy: The FY 2012 budget estimate for the Lung Diseases program is $639.519 million, an increase of $9.581 million or 1.52% over the FY 2010 actual. In FY 2012 the program will continue to support the initiative to identify the fundamental etiologic, pathologic, and genetic commonalities between lung cancer and chronic obstructive pulmonary disease in order to characterize the determinants of individual susceptibility and the shared biochemical and immunological pathways involved in the origin and progression of the two diseases. In FY 2012 the program plans to support a training initiative to increase the pool of medical students interested in an academic career in pediatric, sleep, or hematology/transfusion medicine.
|
Portrait of a Program: New Directions in Chronic Obstructive Pulmonary Disease (COPD) The NHLBI COPD program seeks to elucidate the pathways by which the disease develops and progresses and to develop effective approaches for COPD management and prevention. Investigators are exploring mechanisms of injury and repair in the lung and environmental and genetic determinants of COPD. Applied studies are developing new methods of lung imaging and using them to characterize various subtypes of COPD and are measuring disease progression and outcomes. The Learn More, Breathe Better campaign seeks to increase awareness that COPD is a serious, but treatable, lung disease and to encourage people at risk to have their breathing tested and talk to their doctors about treatment options. During FY 2011, the NHLBI initiated a new program that brings together researchers from the pulmonary and cancer communities to investigate the common pathogenetic mechanisms of lung cancer and COPD. By studying the genotypic and phenotypic characteristics of individuals with susceptibility to both diseases and the shared biochemical and immunological pathways involved, it is hoped that scientists will understand the fundamental processes that regulate the origin and progression of both diseases. FY 2011 marks the final year of support for NHLBI Specialized Centers of Clinically Oriented Research in COPD. In FY 2012 the NHLBI will fund the design and testing of a strategy for finding cases of moderate-to-severe COPD, with the ultimate goal of prompt and effective application of proven treatments to reduce morbidity and mortality. A second initiative will support identification of early molecular abnormalities associated with alpha-1 antitrypsin deficiency in affected persons with or without clinical lung disease. The Institute also plans to establish the Lung Repair and Regeneration Consortium to develop new research tools, reagents, and models to elucidate the lung’s capacity for regeneration. |
Blood Diseases and Resources
This program supports research on the causes, prevention, and treatment of nonmalignant blood diseases, including anemias, sickle cell disease, and thalassemia; premalignant processes such as myelodysplasia and myeloproliferative disorders; abnormalities of hemostasis and thrombosis such as hemophilia; and immune dysfunction. Another program responsibility is to support research and research training on the use, safety, efficacy, and availability of blood and blood components for transfusion and cellular therapeutics. In FY 2010, NHLBI continued support for the landmark Clarification of Optimal Anticoagulation through Genetics (COAG) clinical trial of genotype-guided dosing of warfarin therapy. In FY 2011, NHLBI will renew the Blood and Marrow Transplant Clinical Trials Network and fund new planning grants for pivotal clinical trials in hemoglobinopathies.
Budget Policy: The FY 2012 budget estimate for the Blood Diseases and Resources program is $395.091 million, an increase of $5.919 million or 1.52% over the FY 2010 actual. The program plans for FY 2012 include continued support to accelerate research on the management of hematopoietic stem cell transplantation, standardize and compare the effectiveness and toxicities of existing treatments, and evaluate new therapies. Another planned initiative will provide investigators with the resources (i.e., funding, biospecimens and associated data), to conduct exploratory research in heart, lung, and blood diseases, and blood resources. This program will provide investigators with an opportunity to obtain preliminary data using human biospecimens in a timely and cost effective manner. These data can be used in future grant applications to advance the mission of NHLBI.
Portrait of a Program: Translational Research Centers in Thrombotic and Hemostatic Disorders The TRC-THD program is designed to accelerate the translation of basic research discoveries into improved approaches for prevention, diagnosis, and treatment of thrombotic and hemostatic disorders. Early-stage translation research will integrate applied and basic science to move scientific discoveries toward clinical application. Collaboration between the new centers and NIH Clinical and Translational Science Award programs or institutional programs with similar capabilities is expected to leverage resources and facilitate navigation of regulatory obstacles. The NHLBI anticipates funding five centers, each of which would address a different clinical question. Expected outcomes of this endeavor are the identification of predictive markers of risk, development of improved diagnostic tools, and discovery of new therapeutic agents. This new program supplants the NHLBI Specialized Centers of Clinically Oriented Research (SCCOR) in Hemostatic and Thrombotic Diseases program, which—although highly productive—did not emphasize early-stage translational research and ended in FY 2011. |
Intramural Research
The NHLBI Intramural Research Program (IRP) conducts laboratory and clinical research in heart, vascular, lung, blood, and kidney diseases and develops technology related to cardiovascular and pulmonary diseases. These studies extend from the basic structure and interaction of proteins and motility/energetics of cells to the clinical diagnosis and treatment of a wide range of diseases. The program comprises six centers (Biochemistry and Biophysics, Cell Biology and Physiology, Genetics and Developmental Biology, Immunology, Molecular Medicine and Systems Biology) and two branches (Hematology, Cardiovascular and Pulmonary). In FY 2010, the NHLBI IRP continued its contributions to two trans-NIH programs—the Center for Human Immunology and the Imaging Probe Development Center—which are administered and staffed by the NHBLI for the entire NIH campus. The IRP conducted the first-ever Earl Stadtman Tenure Track search at the NIH level to recruit the next generation of scientists. In fiscal year 2011, the program on Pediatric Imaging using MRI, in collaboration with the Children’s National Medical Center, will start seeing pediatric cases. Its goals are to minimize ionizing radiation exposure, apply real-time MRI approaches to simple diagnostic procedures that avoid general anesthetics, and provide visual guides for minimally invasive surgery. The program will complement a large effort in cardiovascular imaging, both on campus and at Suburban Hospital that seeks to improve diagnosis and treatment of heart disease. The IRP is recruiting a new director for the Systems Biology Center who will be expected to develop a dynamic, highly productive program that can integrate with similar efforts across the NIH and provide national and international leadership in selected areas.
Budget Policy: The FY 2012 budget estimate for the Intramural Research program is $190.900 million, an increase of $5.596 million or 3.0% from the FY 2010 actual. Resources will support ongoing research and modest expansion of new programs.
Research Management and Support
This activity provides administrative management and scientific direction in the review, award, and monitoring of research grants, training awards, and research and development contracts and in the overall planning, coordination, and evaluation of the Institute’s programs. The Division for the Application of Research Discoveries (DARD) continued its We Can! ® Program, which addresses overweight and obesity in children 8 through 13 years of age. In FY 2010, We Can! ® and its partners held nine instructional regional trainings for over 700 participants, who will bring the program into their communities across the country. In FY 2011, the DARD plans to release and begin to implement pediatric integrated cardiovascular risk reduction guidelines, complete and release sickle cell disease guidelines, and release clinical guidelines on hypertension, cholesterol, and obesity. The Office of Communications (OC) provides comprehensive, integrated, and technology-supported communications abilities to help the NHLBI achieve its mission. In FY 2010, the OC focused NHLBI efforts to celebrate a century of progress in sickle cell disease research and care through briefings, public health information, and coalition-building activities to prepare partners and the sickle cell community for the abovementioned release of sickle cell disease guidelines. In FY 2011, the OC will begin implementing the Institute’s new Web strategies, integrating a new content management system and full information architecture redesign to improve public access to research and health information.
Budget Policy: The FY 2012 budget estimate for Research Management and Support is $123.861 million, an increase of $4.217 million or 3.5% over the FY 2010 actual.
Budget Authority by Object
(Dollars in Thousands)
|
Includes FTEs which are reimbursed from the NIH Common Fund for Medical Research
Salaries and Expenses
(Dollars in Thousands)
| OBJECT
CLASSES |
FY 2010 Actual |
FY 2012 PB |
Increase
or Decrease |
Percent Change |
|---|---|---|---|---|
Personnel Compensation: |
||||
| Full-time permanent (11.1) | $60,342 | $61,638 | $1,296 | 2.1 |
| Other than full-time permanent (11.3) | 32,096 | 32,764 | 668 | 2.1 |
| Other personnel compensation (11.5) | 3,808 | 3,890 | 82 | 2.2 |
| Military personnel (11.7) | 783 | 824 | 41 | 5.2 |
| Special personnel services payments (11.8) | 8,187 | 8,356 | 169 | 2.1 |
| Total Personnel Compensation (11.9) | 105,216 | 107,472 | 2,256 | 2.1 |
| Civilian personnel benefits (12.1) | 25,706 | 26,250 | 544 | 2.1 |
| Military personnel benefits (12.2) | 535 | 546 | 11 | 2.1 |
| Benefits to former personnel (13.0) | 0 | 0 | 0 | 0.0 |
| Subtotal, Pay Costs | 131,457 | 134,268 | 2,811 | 2.1 |
| Travel (21.0) | 3,411 | 3,663 | 252 | 7.4 |
| Transportation of things (22.0) | 276 | 296 | 20 | 7.2 |
| Rental payments to others (23.2) | 3 | 3 | 0 | 0.0 |
| Communications,
utilities and miscellaneous charges (23.3) |
1,058 | 1,137 | 79 | 7.5 |
| Printing and reproduction (24.0) | 261 | 281 | 20 | 7.7 |
| Other Contractual Services: | ||||
| Advisory and assistance services (25.1) | 2,139 | 2,265 | 126 | 5.9 |
| Other services (25.2) | 33,409 | 34,995 | 1,586 | 4.7 |
| Purchases from government accounts (25.3) | 197,580 | 198,271 | 691 | 0.3 |
| Operation and maintenance of facilities (25.4) | 2,086 | 2,239 | 153 | 7.3 |
| Operation and maintenance of equipment (25.7) | 4,239 | 4,551 | 312 | 7.4 |
| Subsistence and support of persons (25.8) | 0 | 0 | 0 | 0.0 |
| Subtotal Other Contractual Services | 239,453 | 242,321 | 2,868 | 1.2 |
| Supplies and materials (26.0) | 14,286 | 15,333 | 1,047 | 7.3 |
| Subtotal, Non-Pay Costs | 258,748 | 263,034 | 4,286 | 1.7 |
| Total, Administrative Costs | 390,205 | 397,302 | 7,097 | 1.8 |
Details of Full-Time Equivalent Employment (FTEs)
OFFICE/DIVISION |
FY 2010 Actual |
FY 2011 CR |
FY 2012 PB |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Civilian | Military | Total | Civilian | Military | Total | Civilian | Military | Total | |
| Office of the Director | 99 | 1 | 100 | 100 | 1 | 101 | 100 | 1 | 101 |
| Division of Blood Diseases and Resources | 25 | 25 | 25 | 0 | 25 | 25 | 0 | 25 | |
| Division of Lung Diseases | 26 | 26 | 26 | 0 | 26 | 26 | 0 | 26 | |
| Division for the Application of Research Discoveries | 24 | 24 | 24 | 0 | 24 | 24 | 0 | 24 | |
| Division of Intramural Research | 448 | 7 | 455 | 450 | 6 | 456 | 450 | 6 | 456 |
| Division of Cardiovascular Sciences | 124 | 1 | 125 | 124 | 1 | 125 | 124 | 1 | 125 |
| Division of Extramural Research Activities | 121 | 0 | 121 | 121 | 0 | 121 | 121 | 0 | 121 |
| Total | 867 | 9 | 876 | 870 | 8 | 878 | 870 | 8 | 878 |
Includes FTEs which are reimbursed from the NIH Common Fund for Medical Research. |
0 | 0 | |||||||
FISCAL
YEAR |
Average
GM/GS Grade |
|---|---|
2008 |
12.2 |
2009 |
12.4 |
2010 |
12.4 |
2011 |
12.4 |
2012 |
12.4 |
Detail of Positions
GRADE |
FY 2010 Actual |
FY 2011 CR |
FY 2012 PB |
|---|---|---|---|
| Total, ES Positions | 0 | 0 | 0 |
| Total, ES Salary | 0 | 0 | 0 |
| GM/GS-15 | 96 | 96 | 96 |
| GM/GS-14 | 136 | 138 | 138 |
| GM/GS-13 | 161 | 161 | 161 |
| GS-12 | 91 | 91 | 91 |
| GS-11 | 46 | 46 | 46 |
| GS-10 | 1 | 1 | 1 |
| GS-9 | 53 | 53 | 53 |
| GS-8 | 25 | 25 | 25 |
| GS-7 | 12 | 12 | 12 |
| GS-6 | 6 | 6 | 6 |
| GS-5 | 6 | 6 | 6 |
| GS-4 | 2 | 2 | 2 |
| GS-3 | 1 | 1 | 1 |
| GS-2 | 0 | 0 | 0 |
| GS-1 | 0 | 0 | 0 |
| Subtotal | 636 | 638 | 638 |
| Grades established
by Act of July 1, 1944 (42 U.S.C. 207): |
|||
| Assistant Surgeon General | 1 | 1 | 1 |
| Director Grade | 5 | 4 | 4 |
| Senior Grade | 1 | 1 | 1 |
| Full Grade | 2 | 2 | 2 |
| Senior Assistant Grade | 0 | 0 | 0 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 9 | 8 | 8 |
| Ungraded | 296 | 297 | 297 |
| Total permanent positions | 642 | 642 | 642 |
| Total positions, end of year | 941 | 943 | 943 |
| Total full-time equivalent (FTE) employment, end of year | 876 | 878 | 878 |
| Average ES salary | 0 | 0 | 0 |
| Average GM/GS grade | 12.4 | 12.4 | 12.4 |
| Average GM/GS salary | 101,662 | 101,662 | 101,662 |
New Positions Requested
| FY 2012 | |||
|---|---|---|---|
| Grade | Number | Annual Salary |
|
| Health Science Administrator | GS-14 | 1 | $120,907 |
| Clinical Trials Specialist | GS-14 | 1 | $120,907 |
| Total Requested | 2 | $241,814 | |
Last Updated February 2011
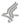


















 Twitter
Twitter
 Facebook
Facebook YouTube
YouTube