Medical Devices
General Human Factors Information and Resources
What is Human Factors/Usability Engineering?
Human factors/usability engineering focuses on the interactions between people and devices. The critical element in these interactions is the device user interface, depicted as the green zone in the figure below.
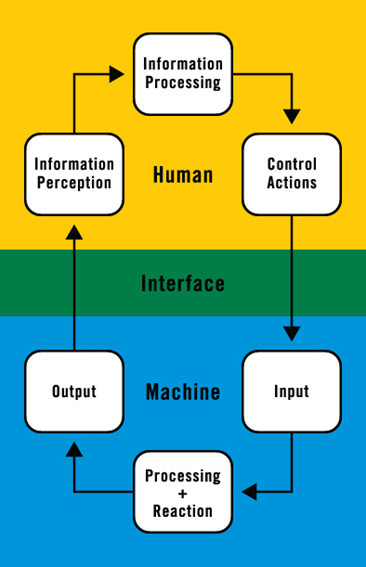
Flow chart adapted from Redmill and Rajan (1997).
To understand the human-machine system, it’s important to understand the ways that people:
- Perceive information from the device,
- Interpret the information and make decisions about what to do, and
- Manipulate the device, its components, and/or its controls.
It’s also important to understand the ways that devices:
- React to input from the user, and then
- Provide feedback to the user about the effects of their actions.
Human factors/usability engineering is used to design the machine-human (device-user) interface. The user interface includes all components with which users interact while preparing the device for use (e.g., unpacking, set up, calibration), using the device, or performing maintenance (e.g., cleaning, replacing a battery, making repairs).
Why is HFE important to medical devices?
For medical devices, the most important goal of the human factors/usability engineering process is to minimize use-related hazards and risks and then confirm that these efforts were successful and users can use the device safely and effectively.
Specific beneficial outcomes of applying human factors/usability engineering to medical devices include:
- Easier-to-use devices,
- Safer connections between device components and accessories (e.g., power cords, leads, tubing, cartridges),
- Easier-to-read controls and displays,
- Better user understanding of the device’s status and operation,
- Better user understanding of a patient’s current medical condition,
- More effective alarm signals,
- Easier device maintenance and repair,
- Reduced user reliance on user manuals,
- Reduced need for user training and retraining,
- Reduced risk of use error,
- Reduced risk of adverse events, and
- Reduced risk of product recalls.
CDRH Human Factors Presentations (Most Recent)
Human Factors Engineering of Combination Products and the FDA (July 2012) (PDF - 332KB)FDA Perspectives on Human Factors in Device Development (June 2012) (PDF - 954KB)FDA Human Factors Draft Guidance Document: Agency Expectations for Human Factors Data in Premarket Submissions (March 2012) (PDF - 659KB)The FDA Perspective on Human Factors in Medical Device Software Development (February 2012) (PDF - 1MB)American Medical Informatics Association (AMIA) Pre-Symposium (Washington, DC) – “FDA Initiatives on Human Factors and Usability for Medical Devices” (October 2011) (PDF - 368KB)Regulatory Affairs Professional Society (RAPS) Annual Conference (Indianapolis, IN) –“Human Factors Considerations for Combination Products” (October 2011) (PDF - 552KB)Meet the Human Factors Pre-market Review Team at FDA’s Office of Device Evaluation, 2011 HFES Annual Meeting (September 2011) (PDF - 1.1MB)- Identifying Use Errors and Human Factors Approaches to Controlling Risks, Public Workshop: Quarantine Release Errors (September 2011) (PDF - 538KB)
Identifying and Mitigating Potential Use Errors (June 2011) (PDF - 215KB)Human Factors/Usability for Medical Devices: An Historical Perspective (June 2011) (PDF - 62KB)Presentation: Enhancing the Quality of Device Labeling - Molly Follette Story, PhD (PDF - 249KB)Presentation: Ron Kaye, MA - "Human Factors / Usability for Infusion Pumps: Additional Test Data Requested In New Draft Guidance" (PDF - 527KB)- AAMI sponsored training: Linking Human Factors with FDA's Quality System Regulation: A Critical Component to the Design and Manufacturing Process (April 25, 2006)

AAMI/FDA Conference "Human Factors in Medical Devices: Design, Regulation, and Patient Safety"
CDRH Human Factors Research Projects (Most Recent)
- UPCARE: An Analysis, Description, and Educational Tool for Medical Device Use Problems (2003)

Report on Medical Device Labeling: Patients' and Lay Caregivers' Medical Device Information and Labeling Needs - Results of Qualitative Research (August 1999) (PDF) (PDF - 86KB)Health Care Practioners' Medical Device Information and Labeling Needs -- Results of Qualitative Research
Additional Medical Device Human Factors Resources (Alphabetical Order)
- Agency for Healthcare Research and Quality (AHRQ): Patient Safety
- Agency for Healthcare Research and Quality (AHRQ): Making Health Care Safer: A Critical Analysis of Patient Safety Practices
- Association for the Advancement of Medical Instrumentation (AAMI): Human Factors for Medical Devices Course

- Center for Universal Design, North Carolina State University

- ECRI Institute: Device Safety

- FAA’s Human Factors Awareness Web Course
- Human Factors and Ergonomics Society (HFES): Health Care Technical Group

- Industrial Designers Society of America (IDSA): Medical Section

- Industrial Designers Society of America (IDSA): Human Interaction Section, DESIGNING for humans

Preventing Medication Errors, Institute of Medicine report (2006) 
- Qmed: Newsletters

- Society for Technical Communication, Usability and User Experience Community: Usability Resources

- Trace Center, University of Wisconsin website on Designing a More Usable World

- Usability Professionals' Association (UPA): Publications

Usability Net