Managing Elevated Blood Lead Levels Among Young Children: Recommendations from the Advisory Committee on Childhood Lead Poisoning Prevention
Chapter 3 - Medical Assessment and Interventions
- Table 3.1. Summary of Recommendations for Children with Confirmed (Venous) Elevated Blood Lead Levels
- Introduction
- General Principles of Medical Case Management
- Medical History
- Table 3.2. Guidelines for Questions to Ask Regarding a Child’s Environmental History
- Physical Examination
- Laboratory and Imaging Evaluation
- Chelation Therapy
- Monitoring Blood Lead Levels
- Table 3.3. Recommended Schedule for Obtaining a Confirmatory Venous Sample
- Table 3.4. Schedule for Follow-Up Blood Lead Testinga
- Monitoring the Child
- Recommendations for Future Research
- References
- Figure 3.1. Lowest Reported Effect Levels of Inorganic Lead in Children
Table 3.1. Summary of Recommendations for Children with Confirmed (Venous) Elevated Blood Lead Levels
| Blood Lead Level (µg/dL) | ||||||
| 10 - 14 | 15 - 19 | 20 - 44 | 45 - 69 | >$70 | ||
|
Lead education -Dietary -Environmental Follow-up blood lead monitoring |
Lead
education Proceed
according to actions for 20-44 µg/dL if: |
Lead education
Complete history
Lab work: Environmental investigation Lead hazard reduction
Neurodevelop-
Abdominal X-ray |
Lead education
Complete history
Lab work: Environmental investigation Lead hazard reduction
Neurodevelop-
Abdominal X-ray Chelation therapy |
Hospitalize and commence chelation therapy Proceed according to actions for 45-69 µg/dL |
||
|
The following actions are
NOT recommended at any blood lead level: |
||||||
|
||||||
Introduction
Case management of children with elevated blood lead levels (EBLLs) requires a different approach from that used in the past. Prior to the development of programs aimed at screening children for EBLLs, lead exposure was generally not detected until a child presented with symptoms of lead toxicity. Neurological findings associated with acute encephalopathy (lethargy, ataxia, seizures, papilledema, and coma) were often the first signs of an EBLL, and children with these symptoms required immediate hospitalization and treatment. Encephalopathy could result from a blood lead level (BLL) >=70 µg/dL and could develop without prior symptoms. Among children with BLLs exceeding 150 µg/dL, laboratory abnormalities often included phosphaturia, proteinuria, aminoaciduria, glucosuria, and hypophosphatemia (1-3).
Today such presentations are rare. Children with EBLLs usually have BLLs below 30 µg/dL, and few BLLs exceed 50 µg/dL. Most children with EBLLs have no symptoms. Case management now focuses on reducing children’s exposure to lead and decreasing their BLLs, whether they have symptoms of lead toxicity or not. What follows is a guide to the basic standards and principles of medical case management. It is not intended for use as a complete protocol but rather as a tool for adapting management to local needs and conditions.
General Principles of Medical Case Management
Coordinating Care
Coordination of care is critical to successful case management. For each child, an individualized plan of follow-up must be devised and implemented. Members of the case management team need to maintain open lines of communication and work together. Case managers and primary care providers (PCPs), in particular, must work collaboratively to ensure proper medical management and follow-up.
Conducting Medical Case Management
Medical case management for children with EBLLs is largely predicated on a secondary prevention model (i.e., intervention after an EBLL has been detected, usually prior to the onset of symptoms). By interrupting the process of lead poisoning through early detection and intervention, case managers working with PCPs can prevent children from dying or suffering severe permanent sequelae of lead toxicity such as persistent seizures and mental retardation (4, 5).
The detrimental effects of EBLLs in the range of 10 to 45 µg/dL are usually subclinical and may include neurodevelopmental impairment often apparent only at a later age. (See Chapter 5, "Developmental Assessment and Intervention.") Figure 3.1 illustrates the lowest reported BLLs for some of the effects associated with EBLLs. If a child presents without symptoms, the child’s PCP and case manager may have trouble convincing the child’s caregiver of the importance of suggested interventions. Case managers should manage each child individually, taking into consideration the child’s BLL and the ability of caregivers to cooperate and implement interventions.
Identifying Childrenwith EBLLs
Screening programs are the main vehicle for identifying children with EBLLs. Those found in this manner typically have BLLs from 10 to 30 µg/dL and present with no abnormalities on routine medical history, physical examination, or laboratory tests (other than their EBLL). It is critical that case managers as well as PCPs not equate the absence of clinical symptoms, physical abnormalities, or abnormal laboratory results with an absence of toxicity.
Identifying Sources of Lead Exposure
When evaluating a child with an EBLL, case managers must identify the sources of a child’s lead exposure. The most common source of lead in children with EBLLs is leaded paint. Housing built before 1950 has been shown to be routinely contaminated with lead and to represent a risk for children (6, 7). Contamination of dust or soil occurs when leaded paint chalks or chips, or is subject to friction. (See Chapter 2, "Assessment and Remediation of Residential Lead Exposure.") Other less common sources include lead in water, lead in substances used in caregiver hobbies or occupations, lead in culturally specific substances such as folk remedies, and lead in imported cookware or cosmetics (Appendix 1).
Medical History
General Considerations
Although abdominal pain, vomiting, constipation, change in appetite, and irritability have been described in association with EBLLs, they are seldom caused by BLLs less than 40 µg/dL, and other causes for such symptoms should be sought. Case managers, caregivers, and PCPs may note increased activity among children with BLLs < 45 µg/dL. However, they should not assume that increased activity is related to the EBLL (5, 8).
History Taking to Determine Lead Sources
A child’s environmental history can provide information about the child’s possible exposure to residential and other sources of lead. It should:
- Include elements specific to the child: ethnic group, caregiver hobbies and occupations, and local lead hazards.
- Be taken from a person who regularly observes the child’s activity and behavior.
- Identify all sites where the child spends significant amounts of time.
- Be obtained independently in both office and home settings by the PCP, case manager, or others involved in the child’s care.
- Be accompanied by a full environmental investigation for all children with BLLs >=20 µg/dL or two venous BLLs >=15 µg/dL at least 3 months apart.
Taking a history in the child’s home allows for direct observation and further in-depth questioning. If a child’s BLL remains elevated despite lead hazard reduction, less common sources should be considered. Because a child may be exposed to lead from multiple sources, identifying one source may not be sufficient to eliminate all lead exposure. Repeated history taking by different members of the management team is often required.
Because knowledge about the lead sources in a community and the prevalence of EBLLs in specific geographic areas of the community can be useful in determining sources of exposure, the interpretation of a child’s environmental history may require consultation with lead experts. Case managers play a crucial role in treating children’s EBLLs by fostering a multi-disciplinary approach to the environmental evaluation and by coordinating communication among public health officials, PCPs, and caregivers. Table 3.2 outlines suggested questions to ask in determining a child’s environmental history. This is only intended to be a guide, and case managers and PCPs are encouraged to tailor this list to local needs.
| Paint
and soil exposure
What is the age and general condition of the residence? Relevant behavioral characteristics of the child To what degree does the child exhibit hand-to-mouth activity? Exposures to and behaviors of household members What are the occupations of adult household members? Miscellaneous questions Does the home contain vinyl mini-blinds made overseas and purchased before
1997? |
Case managers should consider the following elements in assessing a child’s exposure to lead in paint or soil:
Age and condition of housing: Pre-1950 housing that is in poor condition poses the greatest risk for children (6,7). Housing built from 1950 through 1978 may also contain leaded paint, although the concentration of lead in paint was lower during this period than previously. The condition of the home is important: deterioration of lead-painted surfaces markedly increases the risk to children (9-12); water damage from roofing and plumbing can increase peeling and chipping of paint; open windows with debris in the window well provide an additional source of exposure.
Duration of a child’s habitation at that site and whether the child has moved recently: Because leaded dust generally causes EBLLs only after a significant duration of exposure, children with EBLLs who recently moved into their current residence may have been exposed to lead at their prior residence. Conversely, those who have moved from a lead-free or lead-safe environment to a more risky one are more likely to need ongoing active surveillance.
Whether the residence has been renovated: Any disturbance of leaded paint, including repairs, renovation, or improper lead abatement can result in generation of leaded dust.
Other possible exposure locations: It is important to document locations where a child with an EBLL spends considerable periods of time, such as relatives’ or babysitters’ houses. Such locations may be a source of exposure and therefore should be subjected to the same scrutiny as the primary habitation.
Character of indoor play areas: Do play areas have lead hazards? Are there window wells, windowsills, or other painted edges near indoor play areas? These potential sources of leaded dust or paint may be subject to chewing or mouthing behaviors typical of young children.
Soil exposure: In outdoor areas, the most heavily contaminated soil is adjacent to the house, particularly in the drip line. Soil contamination may also occur from industrial sites and past automobile emissions, particularly along heavily traveled thoroughfares.
Dust and dirt control: Children’s BLLs declined when a team of professionals thoroughly and regularly cleaned the children’s homes (13). At present, there is no evidence that routine house cleaning by family members or frequent and thorough hand washing decreases BLLs. However, because leaded dust is a primary contributor to EBLLs (14-17), strict attention to house cleaning and preventing dust accumulation may be protective. (See Chapter 6, "Educational Interventions for Caregivers.")
Relevant child behaviors
While pica has long been a known risk factor for EBLLs, typical hand-to-mouth activity during the toddler years is a more frequent cause of lead ingestion (18, 19). Although no supporting data are available, frequent hand washing may help lower EBLLs of young children.
Caregiver exposures and at-risk behaviors
Household members who work in lead-contaminated environments or participate in certain hobbies can bring lead into the home on their clothing or shoes (20). It is important to ask such household members whether they regularly shower and change clothes and shoes after these activities. Caregivers may also introduce airborne lead into the home by burning lead-contaminated materials in an indoor fireplace. (See Appendix I for a detailed discussion of caregiver exposures.)
Miscellaneous
Water: When no other source of lead is found, the water supply should be considered. (See Chapter 2, "Assessment and Remediation of Residential Lead Exposure.") While public water systems must monitor, and, if necessary, treat tap water for elevated lead levels, regulations governing lead in drinking water do not apply to households supplied by private wells. Municipal water companies and private industrial laboratories can advise case managers and caregivers on how to collect and process water. In areas where there are no known hazardous water-supply lines, lead contamination of the water may occur within the home from lead solder in plumbing fixtures or from fixtures made of lead-containing alloys. Contamination is increased when the water is relatively acidic (pH < 6.5). Moore found that water lead levels and the subsequent BLLs of children drinking such water substantially decreased when the pH of drinking water was raised to > 8.5. He also found that low mineral content increased lead contamination in water, but not as greatly as acidity (21). Water that is hot or has been stationary in pipes overnight contains more lead than freely running cold water. Lead contamination decreases with the aging of home plumbing, particularly if the water supply is alkaline or contains significant calcium or other mineral deposits (22, 23).
Mini-blinds: Imported vinyl mini-blinds may contain lead and result in exposure. (See Appendix I.)
Cultural practices: Specific ethnic groups may use imported folk remedies, cosmetics, food, or cookware contaminated with lead. Practices resulting in lead exposure from these sources are often localized, and determining children’s risk from such practices requires knowledge about a specific group’s cultural habits. Caregivers may be reluctant to admit using some of these items, and it is important not to put them on the defensive when taking the history. (See Appendix I.)
Newly identified sources: In the past, various items have emerged as lead hazards, often first presented through the news media. Case managers and PCPs should be cognizant of recent media and Consumer Product Safety Commission reports that may be relevant to children with whom they have contact (see http://www.cpsc.gov).
Physical Examination
Children evaluated as a consequence of an EBLL found by screening most often have no physical findings specific for lead toxicity. Gingival lead lines, although often stressed during medical training, are rarely seen in clinical practice and are of no use in the diagnosis and management of children with EBLLs. Pallor, papilledema, and other neurologic findings suggestive of acute encephalopathy would not be expected.
A thorough evaluation of all children with BLLs >=20 µg/dL is recommended for three reasons. First, it will allow PCPs to ascertain whether children with such EBLLs have any findings suggestive of encephalopathy. The BLL threshold for encephalopathic findings is believed to be 70 µg/dL, although encephalopathy is usually associated with much higher BLLs (1, 3). Second, it will allow PCPs to assess whether children with EBLLs are engaging in at-risk behaviors such as pica and hand-to-mouth activity. Finally, it will allow PCPs to identify behavioral and neurodevelopmental disorders, such as distractibility, aggression, or speech delay. If a child has any of these findings, regardless of their etiology, the case manager or PCP should, when appropriate, refer the child for a further evaluation. (See Chapter 5, "Developmental Assessment and Interventions.")
Laboratory and Imaging Evaluation
Among asymptomatic children, most clinical laboratory results other than BLLs will be normal and therefore will not be of assistance in case management. However, all children should have a hemoglobin or hematocrit test performed, as anemia is associated with EBLLs. That iron deficiency rather than lead is the cause of such anemia does not diminish the need for follow-up (24, 25). PCPs may wish to assess children’s iron stores by one or more of a variety of laboratory tests. Iron deficiency may delay children’s neurodevelopment independently of the effects of lead (26). Because basophilic stippling is not specific for lead toxicity, a peripheral blood smear is of no use in the hematologic evaluation.
The inhibition of heme synthesis leads to the accumulation of excess porphyrins, particularly protoporphyrin IX in red cells. The fluorescence of these porphyrins has led to the development of methods to detect extracted porphyrins—free erythrocyte protoporphyrin (FEP) or erythrocyte porphyrin (EP) testing—and to the observation of zinc protoporphyrin (ZPP) in red cells. Because the relationship between the results of these tests and BLLs is log-linear, these tests can be used to evaluate and follow children with very high BLLs. However, the results are confounded by concomitant iron deficiency and show poor correlation with BLLs <=25 µg/dL (27). Therefore, EP tests should be used infrequently except in evaluating children with BLLs well above 25 µg/dL whose BLLs do not show a steady decline in response to medical and environmental interventions. In such situations, these measures may assist PCPs in differentiating BLL rebound after treatment from the effects of re-exposure.
While very high BLLs have been associated with serious to severe renal tubular dysfunction (2, 28), there is no evidence to support routinely evaluating the renal status of children with presymptomatic BLLs. However, if potentially nephrotoxic chelating agents such as EDTA are to be used in treatment, renal function testing is appropriate prior to and during therapy.
Abdominal radiographs may be useful in determining whether children are currently ingesting lead-contaminated non-food items, including paint chips. They are particularly useful when children have an unexpected acute rise in BLL or are not responding to case management as expected. Long-bone films for the presence of growth arrest lines ("lead lines") may be of interest but rarely provide information useful for a child’s case management (29). Lead lines are not present unless BLLs exceed 50 µg/dL and are indicative of chronic exposure (30). Also of no documented utility in the management of children with EBLLs are hair (31), fingernail, and tooth (dentin) lead measurements. Hair and fingernails are subject to external contamination, which makes the results of lead tests on them uninterpretable.
A few studies have demonstrated alteration of neurophysiologic function (e.g., postural sway, auditory evoked potentials, nerve conduction) with BLLs observed today (32-35). However, further research is needed to define normative standards and determine inter-individual variation and clinical significance. Until then, such measures are of little use in the diagnosis or management of an individual child.
X-ray fluorescence of long bones uses a radioactive source to provide noninvasive estimation of lead in bone (36). At the present time, it should be considered a research tool to be used only to characterize groups of children in epidemiological studies. As with the neurophysiologic methods discussed previously, it is insufficiently standardized, and results show significant inter-laboratory variation.
Chelation Therapy
While chelation therapy is considered a mainstay in the medical management of children with BLLs > 45 µg/dL, it should be used with caution. Primary care providers should consult with an expert in the management of lead chemotherapy prior to using chelation agents. If unaware of a center with such expertise, PCPs should contact their local or state lead poisoning prevention program, local poison control center, or the Lead Poisoning Prevention Branch at CDC (404-498-1420) for the names of accessible experts. A child with an EBLL and signs or symptoms consistent with encephalopathy should be chelated in a center capable of providing appropriate intensive care services!
Controversy exists as to the appropriate level at which to initiate chelation therapy, and which drugs are most appropriate. Succimer treatment of young children with BLLs < 45 µg/dL lowered their BLLs but failed to improve their neurodevelopmental test scores (37). (See Chapter 5, "Developmental Assessment and Interventions.") Chelation therapy with succimer is addressed in a document on pharmaceutical agents in the treatment of lead poisoning (38).
If oral outpatient chelation therapy is undertaken, the case manager should ensure that caregivers adhere to the prescribed dosing schedule and should serve as the liaison between the medical community and the child’s caregiver. Treatment should occur in a lead-safe environment.
Monitoring Blood Lead Levels
Measurement of BLLs is the main method of determining whether significant absorption of lead has occurred, how urgently intervention is needed, and how successful case management has been. When a child’s BLL does not fall within a reasonable amount of time, it is the responsibility of the case manager and other team members to determine the cause of failure. The rate of BLL decrease can depend on both the amount of lead in the child’s body and the duration of the BLL elevation. A course of chelation therapy with succimer results in a rapid fall in BLL after 1 week of treatment. However, BLLs of those treated rebound after treatment ends, and by approximately 7 weeks after an initial course of therapy, BLLs of treated patients may reach almost 75% of prechelation levels (39). CDC recommends rechecking children’s BLLs 7 to 21 days after completion of chelation therapy (40). A continuing increase in children’s BLLs above the rebound level during the follow-up period may indicate continuing or possibly increased exposure to lead and definitely indicates a need for further environmental investigation. Common causes of rising BLLs include failure to address hazards in the child’s environment, improper environmental lead abatement techniques, and continued use of imported pottery, cosmetics, or folk medicines that are contaminated with lead. However, medical conditions resulting in bed rest or similar immobilization (41), or in acidosis (42), can cause children’s BLLs to rise unexpectedly, or fail to fall.
Confirmation of BLL by Venous Sample
Any screening BLL above 10 µg/dL must be confirmed with a venous sample. The time frame for confirmation depends upon the initial BLL (Table 3.3). In general, the higher the screening BLL, the sooner the confirmatory test. However, if a child is less than 12 months old, or if there is reason to believe that the BLL is rising rapidly, an earlier diagnostic confirmation may be indicated.
Table 3.3. Recommended Schedule for Obtaining a Confirmatory Venous Sample
| Screening test result (µg/dL) |
Perform a confirmation test within: |
| 10-19 |
3 months |
| 20-44 |
1 week-1 montha |
| 45-59 |
48 hours |
| 60-69 |
24 hours |
| > 70 |
Immediately as an emergency lab test |
Follow-Up Venous Blood Lead Testing
Medical management includes follow-up blood lead testing. Table 3.4 presents the suggested frequency of follow-up tests. This table is to be used as guidance. Case managers and PCPs should consider individual patient characteristics and caregiver capabilities and adjust the frequency of follow-up tests accordingly.
Table 3.4. Schedule for Follow-Up Blood Lead Testing a
| Venous blood lead level (µg/dL) | Early follow-up (first 2-4 tests after identification) | Late follow-up (after BLL begins to decline) |
| 10-14 | 3 monthsb | 6-9 months |
| 15-19 | 1-3 monthsb | 3-6 months |
| 20-24 | 1-3 monthsb | 1-3 months |
| 25-44 | 2 weeks-1 month | 1 month |
| > 45 | As soon as possible | Chelation with subsequent follow-up |
aSeasonal variation of BLLs exists and may be more apparent in colder climate areas. Greater exposure in the summer months may necessitate more frequent follow ups.
bSome case managers or PCPs may choose to repeat blood lead tests on all new patients within a month to ensure that their BLL level is not rising more quickly than anticipated.
Monitoring the Child
Managing a Child’s Nutrition
Although the effectiveness of nutritional interventions has not been established, the following recommendations are common sense and are appropriate advice for all children, including those with EBLLs:
Consume adequate amounts of bioavailable calcium and iron.
Consume at least two servings daily of foods high in vitamin C, such as fruits, vegetables, and juices.
Eat in areas that pose a low risk for lead exposure; for example, at a table rather than on the floor.
Participate in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) if the family is eligible.
Dietary information is discussed in detail in Chapter 4, "Nutritional Assessment and Interventions."
Educating Caregivers
Educating caregivers is an important part of case management. Caregivers need to understand EBLLs and the risks that an EBLL poses to their child, what they can do to eliminate their child’s exposure to lead, and the importance of follow-up. It is important to not overburden caregivers and to provide them with understandable information and manageable interventions. (See Chapter 6, "Educational Interventions for Caregivers," for a detailed discussion.)
Monitoring a Child’s Developmental Progress
Follow-up also requires attention to the behavioral sequelae of EBLLs. Neurodevelopmental monitoring should continue long after a case meets BLL closure criteria, as many deficits will not manifest themselves until after a child starts school. Because developmental history and testing at the time of an EBLL usually will not identify lead-caused problems, a child’s EBLL history should be part of his or her permanent medical record. A referral for testing of intellectual and behavioral performance, whether or not related to EBLLs, should be made if indicated. (See Chapter 5, "Developmental Assessment and Interventions," for details.) The PCP and case manager should be intimately involved with any educational and behavioral interventions, in consultation with developmental and behavioral experts.
Monitoring Caregiver Compliance with Follow-Up Measures
For many reasons, caregivers may have trouble adhering to follow-up measures. Case managers, PCPs, and other members of the case management team must be careful not to blame the caregivers but should continue to make them aware that follow-up is for the benefit of the child. Caregivers may have trouble appreciating the importance of follow-up for asymptomatic children. Many caregivers have problems with basic needs such as transportation, food, or paying monthly bills. Therefore, it is important to limit interventions to those most likely to benefit the child while being within the capabilities of the caregiver. Punitive interventions, such as referring children to protective services, should be done as a last resort, when all more constructive approaches have been exhausted. Members of the case management team should always remember that virtually all caregivers are doing the best they can for their children and should be assisted in their efforts.
Recommendations for Future Research
Develop improved screening methods through the use of geographic information systems.
Compare succimer with edetate calcium disodium in the treatment of children with EBLLs.
Assess the benefits of hand washing and other low-intensity educational interventions.
Identify primary prevention interventions that are effective in reducing lead exposure.
Establish an effective approach to referral and intervention for children with EBLLs who are suspected of having developmental or behavioral problems.
Evaluate the effectiveness of dietary interventions.
Develop and refine additional screening questions for an effective "environmental checklist."
Determine an appropriate endpoint for completion of chelation therapy.
References
- Chisolm JJ, Harrison HE. The treatment of acute lead encephalopathy in children. Pediatrics 1957;19:2-20.
- Chisolm JJ. Aminoaciduria as a manifestation of renal tubular injury in lead intoxication and a comparison with patterns of aminoaciduria seen in other diseases. J Pediatr 1962;60:1-17.
- Chisolm JJ. The use of chelation agents in the treatment of acute and chronic lead intoxication in childhood. J Pediatr 1968;73:1-38.
- Byers RK, Lord EE. Late effects of lead poisoning on mental development. Am J Dis Child 1943;66:471-94.
- Perlstein MA, Attala R. Neurologic sequelae of plumbism in children. Clin Pediatr 1966;5:292-98.
- Sargent JD, Bailey A, Simon P, et al. Census tract analysis of lead exposure in Rhode Island children. Environ Res 1997;74:159-68.
- CDC. Update: blood lead levels—United States, 1991-1994. MMWR 1997;46:141-6.
- Committee on Measuring Lead in Critical Populations. Measuring Lead Exposure in Infants, Children, and Other Sensitive Populations. National Research Council Commission on Life Sciences, Washington, DC: National Academy Press, 1993. Available at http://books.nap.edu/books/030904927X/html/R1.html#. Accessed 12/19/01.
- Chisolm JJ, Mellits ED, Quaskey SA. The relationship between the level of lead absorption in children and the age, type, and condition of housing. Environ Res 1985;38:31-45.
- Clark CS, Bornschein RL, Succop S, et al. Condition and type of housing as an indicator of potential environmental lead exposure and pediatric blood lead levels. Environ Res 1985;38:46-53.
- Sargent JD, Brown MJ, Freeman JL, et al. Childhood lead poisoning in Massachusetts communities: its association with sociodemographic and housing characteristics. Am J Public Health 1995; 85:528-34.
- Bronson MA, Tilden RL, Renier CM. Community-based screening for childhood lead poisoning. Identification of risk factors and susceptible populations in Duluth. Minn Med 1999;82:25-9.
- Rhoads GG, Ettinger AS, Weisel CP, et al. The effect of dust lead control on blood lead in toddlers: a randomized trial. Pediatrics 1999;103(3):551-5.
- Charney E, Sayre J, Coulter M. Increased lead absorption in inner city children: where does the lead come from? Pediatrics 1980;65:226-31.
- Bornschein RL, Succop P, Kraft KM, et al. Exterior surface dust lead, interior house dust lead and childhood lead exposure in an urban environment. In Hemphill DD (ed). Trace Substances in Environmental Health, XX. Proceedings of University of Missouri’s 20th Annual Conference, June 1986. University of Missouri, Columbia, Missouri, 1987.
- Lanphear BP, Burgoon DA, Rust SW, et al. Environmental exposures to lead and urban children’s blood lead levels. Environ Res 1998;76:120-30.
- Lanphear BP, Matte TD, Rogers J, et al. The contribution of lead-contaminated house dust and residential soil to children’s blood lead levels. Environ Res 1998a:79:51-68.
- Freeman NC, Ettinger A, Berry M, et al. Hygiene- and food-related behaviors associated with blood lead levels of young children in lead contaminated homes. J Expo Anal Environ Epidemiol 1997;7:103-18.
- Duggan MJ. Contribution of lead in dust to children’s blood lead. Environ Health Perspect 1983;50:370-81.\
- Roscoe RJ, Gittleman J, Deddens JA, et al. Blood lead levels among children of lead-exposed workers: a meta-analysis. Am J Indust Med 1999;36:475-481.
- 21. Moore MR. Influence of acid rain upon water plumbosolvency. Environ Health Perspect 1985;63:121-6.
- 22. Agency for Toxic Substances and Disease Registry. The Nature and Extent of Lead Poisoning in Children in the United States: A Report to Congress. Atlanta, Georgia: US Department of Health and Human Services, Public Health Service; 1988.
- Environmental Protection Agency. Reducing lead in drinking water: A Benefit Analysis. Washington, DC: US Environmental Protection Agency, Office of Policy Planning and Evaluation; 1986. EPA-230-09-86-019.
- Serwint JR, Damokoab A, Berger OG, et al. No difference in iron status between children with low and moderate lead exposure. J Pediatr 1999;135:108-10.
- Carvalho FM, Barreto ML, Silvany-Neto AM, et al. Multiple causes of anaemia amongst children living near a lead smelter in Brazil. Sci Total Environ 1984;35:71-84.
- Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. N Engl J Med 1991;325:687-94.
- McElvaine MD, Orbach HG, Binder S, et al. Evaluation of the erythrocyte protoporphyrin test as a screen for elevated blood lead levels. J Pediatr 1991:119:548-50.
- Goyer RA. The renal tubule in lead poisoning. Mitochondrial swelling and aminoaciduria. Lab Invest 1968;19:71-7.
- Sachs HK. The evolution of the radiologic lead line. Radiology 1981;139:81-5.
- 30. Blickman JG, Wilkinson RH, Graef JW. The radiologic "lead band" revisited. Am J Roentgenology 1986;146:245-7.
- Esteban E, Rubin CH, Jones RL, et al. Hair and blood as substrates for screening children for lead poisoning. Arch Environ Health 1999;54:436-40.
- Bhattacharya A, Shukla R, Dietrich K, et al. Effect of early lead exposure on children’s postural balance. Develop Med Child Neurol 1995;37:861-78.
- Otto D, Robinson G, Baumann S, et al. Five-year follow-up study of children with low-to-moderate lead absorption: electrophysiological evaluation. Environ Res 1985;38:168-86.
- Osman K, Pawlas K, Schutz A, et al. Lead exposure and hearing effects in children in Katowice, Poland. Environ Res 1999;80:1-8.
- Landrigan PJ, Baker EL, Feldman RG, et al. Increased lead absorption with anemia and slowed nerve conduction in children near a lead smelter. J Pediatr 1976;89:904-10.
- Hu H, Milder FL, Burger DE. X-ray fluorescence: issues surrounding the application of a new tool for measuring burden of lead. Environ Res 1989;49:295-317.
- Rogan WJ, Dietrich KN, Ware JH, et al. Succimer chelation and neuropsychological development in lead-exposed children. N Engl J Med 2001;344:1421-6.
- American Academy of Pediatrics Committee on Drugs. Treatment guidelines for lead exposure in children. Pediatrics 1995;96:155-60.
- Treatment of Lead-Exposed Children (TLC) Trial Group. Safety and efficacy of succimer in toddlers with blood lead levels of 20-44 µg/dL. Pediatr Res 2000;48:593-9.
- CDC. Preventing lead poisoning in young children. Atlanta, Georgia: US Department of Health and Human Services, CDC; 1991.
- Markowitz ME, Weinberger HL. Immobilization-related lead toxicity in previously lead-poisoned children. Pediatrics 1990;86:455-7.
- Aub JC, Fairhall LT, Minot AS, et al. Lead poisoning. Medicine 1925;4:1-250.
Figure 3.1. Lowest Reported Effect Levels of Inorganic Lead in Children
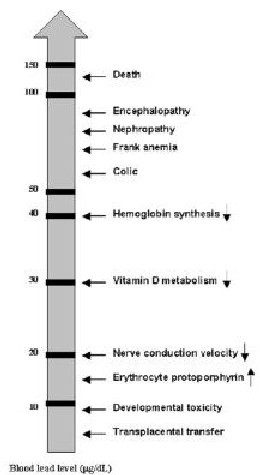
Source: Preventing Lead Poisoning in Young Children, Centers for Disease Control and Prevention; 1991.
Contact Us:
- Centers for Disease Control and Prevention
1600 Clifton Rd
Atlanta, GA 30333 - 800-CDC-INFO
(800-232-4636)
TTY: (888) 232-6348 - cdcinfo@cdc.gov

