|
« Factbook Table of
Contents
10. Research and Development Contracts
NHLBI Research and Development
Contract Obligations*: Fiscal Years 1996–2006
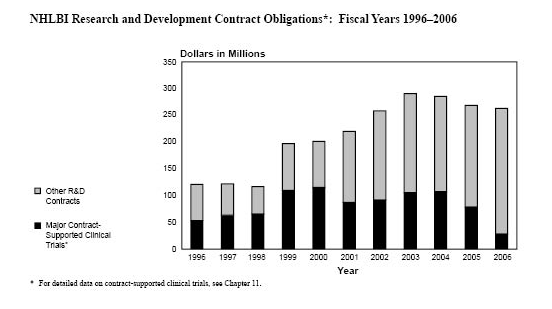
Text-only
With Data Points
* For detailed data on contract-supported clinical
trials, see Chapter 11.
NHLBI Total Research and Development Contract
Obligations: Fiscal Years 1996–2006
| Dollars
(Thousands) |
| Fiscal Year |
| |
1996 |
1997 |
1998 |
1999 |
2000 |
2001 |
2002 |
2003 |
2004 |
2005 |
2006 |
| Heart |
$ 80,373 |
$ 84,820 |
$ 77,886 |
$ 93,270 |
$ 98,715 |
$ 125,291 |
$ 155,971 |
$ 195,425 |
$ 187,043 |
$ 181,970 |
$201,196 |
| Lung |
21,032 |
18,183 |
13,123 |
25,432 |
23,341 |
10,993 |
16,578 |
11,745 |
14,131 |
20,946 |
25,902 |
| Blood |
19,522 |
18,934 |
25,695 |
15,436 |
21,538 |
24,572 |
26,751 |
20,082 |
25,460 |
27,831 |
23,629 |
| Women's Health Initiative |
--- |
--- |
--- |
63,100 |
57,700 |
59,200 |
59,000 |
63,222 |
58,838 |
37,826 |
12,124 |
| Total |
$120,927A |
$121,937B |
$116,704C |
$197,238D |
$201,294E |
$220,056F |
$258,300G |
$290,474H |
$285,472I |
$268,573J |
$262,851K |
A Includes Program Evaluation
Assessment of $4,250,000.
B Includes Program Evaluation and IMPAC II
Assessments of $8,986,000.
C Includes Program Evaluation and IMPAC II
Assessments of $12,589,000.
D Includes Program Evaluation and IMPAC II
Assessments of $14,904,000.
E Includes Program Evaluation and IMPAC II
Assessments of $17,944,000.
F Includes Program Evaluation and IMPAC II
Assessments of $24,579,000.
G Includes Program Evaluation and IMPAC II
Assessments of $35,827,000.
H Includes Program Evaluation and IMPAC II
Assessments of $54,550,000.
I Includes Program Evaluation and IMPAC II
Assessments of $57,545,722.
J Includes Program Evaluation and IMPAC II
Assessments of $64,399,000.
K Includes Program Evaluation and IMPAC II
Assessments of $67,795,000. |
Back to Top
Major NHLBI Research and
Development Contracts by Program
| |
Total
Obligations
Prior to FY 2005 |
Total FY
2006
Obligations |
Total Obligations
to Date |
| Heart and Vascular
Diseases |
|
|
|
|
Atherosclerosis Risk in
Communities (ARIC) |
$121,966,244 |
$6,344,206 |
$128,310,450 |
|
Candidate Gene Association
Research |
--- |
2,789,517 |
2,789,517 |
|
Cardiovascular Health Study
(CHS) |
75,886,177 |
930,000 |
76,816,177 |
|
Coronary Artery Risk
Development in Young Adults (CARDIA) |
71,838,396 |
6,881,765 |
78,720,161 |
|
DNA Resequencing and
Genotyping |
11,000,000 |
7,000,000 |
18,000,000 |
|
Framingham Heart
Study |
62,166,638 |
10,907,043 |
73,073,681 |
|
Genetically Triggered Thoracic
Aortic Aneurysms and Other Cardiovascular Conditions (GENTAC): National
Registry |
--- |
1,391,748 |
1,391,748 |
|
Hispanic Community Health
Study (HCHS) |
--- |
2,900,000 |
2,900,000 |
|
Jackson Heart Study
(JHS) |
20,219,989 |
3,535,576 |
23,755,565 |
|
Multi-Ethnic Study of
Atherosclerosis (MESA) |
54,269,999 |
8,563,705 |
62,833,704 |
|
Pediatric Circulatory
Support |
8,581,202 |
5,959,141 |
14,540,343 |
|
Proteomics
Initiative |
78,893,890 |
18,740,000 |
97,633,890 |
|
Registry for Mechanical
Circulatory Support |
1,215,702 |
1,585,877 |
2,801,579 |
|
SNP Health Association
Research (SHARE) |
--- |
2,000,000 |
2,000,000 |
|
Translational Behavioral
Science Research Consortium |
20,430,569 |
--- |
20,430,569 |
| Lung Diseases |
|
|
|
|
Lung Tissue Research
Consortium |
10,499,994 |
12,598,812 |
23,098,806 |
|
Tuberculosis Curriculum
Coordinating Center |
3,750,000 |
1,125,000 |
4,875,000 |
| Blood Diseases and
Resources |
|
|
|
|
Iron Overload and Hereditary
Hemochromatosis |
25,979,773 |
469,227 |
26,449,000 |
|
Maintenance of Animals for
Hepatitis or AIDS Research |
8,805,530 |
160,474 |
8,966,004 |
|
Maintenance of NHLBI
Biological Specimen Repository |
6,594,342 |
1,031,572 |
7,625,914 |
|
Production of Recombinant B19
Parvovirus Capsids |
2,483,750 |
666,950 |
3,150,700 |
|
Retrovirus Epidemiology Donor
Study (REDS) |
86,865,125 |
8,955,049 |
95,820,174 |
|
Sickle Cell Disease
Health-Related Quality of Life Questionnaire |
584,008 |
708,000 |
1,292,008 |
|
Somatic Cell Therapy
Processing Facilities |
15,576,209 |
6,139,526 |
21,715,735 |
Back to Top
Heart and Vascular Diseases
Program
Atherosclerosis Risk in Communities (ARIC), Initiated
in Fiscal Year 1985
The ARIC program is a large-scale, long-term program
that is measuring associations of CHD risk factors with atherosclerosis by
race, gender, and geographic location. It focuses on early detection of CVD
before symptoms, heart attacks, or strokes occur. The project consists of two
groups: a community surveillance component and a cohort component from four
communities. Three of the cohort components represent the racial mix of their
community, whereas the fourth is exclusively black.
Obligations
Funding History:
Fiscal Year
2006—$6,344,206
Fiscal Years
1985–2005—$121,966,244
Total Funding to
Date—$128,310,450
| Current Active
Organizations and Contract Numbers |
- University of North Carolina at Chapel
Hill
Chapel Hill, North Carolina
|
—HC-55015 |
- Baylor College of Medicine Houston, Texas
Houston, Texas
|
—HC-55016 |
- University of North Carolina at Chapel
Hill
Chapel Hill, North Carolina
|
—HC-55018 |
- University of Minnesota, Twin Cities
Minneapolis, Minnesota
|
—HC-55019 |
- Johns Hopkins University
Baltimore,
Maryland
|
—HC-55020 |
- Mississippi Medical Center
Jackson,
Mississippi
|
—HC-55021 |
- University of Texas Health Science Center
Houston, Texas
|
—HC-55022 |
Candidate Gene Association Resources, Initiated in
Fiscal Year 2006
This program establishes a genotyping and
bioinformatics center to perform high-throughput genotyping for candidate gene
association studies in up to 50,000 participants, and a genome-wide association
study in about 500 disease cases and 1,000 controls. The data will be combined
with available phenotype data to form a genotype-phenotype resource for public
use. DNA for the 50,000-person sample will be collected from multiple NHLBI
cohort studies that have stored samples and available data on a wide array of
heart, lung, blood, and sleep phenotypes.
Obligations
Funding History:
Fiscal Year
2006—$2,789,517
Total Funding to Date—$2,789,517
| Current Active
Organization and Contract Number |
- Massachusetts Institute of Technology
Cambridge, Massachusetts
|
—HC-65226 |
Cardiovascular Health Study (CHS), Initiated in
Fiscal Year 1988
The CHS is a population-based, longitudinal study of
risk factors for development and progression of CHD and stroke in elderly
adults. Extensive data and samples have been collected from nearly 6,000
participants since 1989–1990. The objective for the current CHS:
Transition Phase is to enhance use of the data and samples by the scientific
community during a transition period from a contract-funded, NHLBI-directed
program to one directed by a steering committee of investigators with
independent research support. Seventeen percent of the participants are from
minority populations.
Obligations
Funding History:
Fiscal Year
2006—$930,000
Fiscal Years
1988–2005—$75,886,177
Total Funding to Date—$76,816,177
| Current Active
Organizations and Contract Numbers |
- University of Washington
Seattle,
Washington
|
—HC-55222 |
Coronary Artery Risk Development in Young Adults
(CARDIA), Initiated in Fiscal Year 1984
CARDIA is a long-term study examining the evolution of
CVD risk factors in a cohort of black and white adults, aged 18 to 30 years in
1985–1986. The study examines risk for heart and lung disease and diabetes
by collecting information on body composition, physical activity and lifestyle,
genetics, serologic and metabolic components, inflammatory markers, and other
subclinical markers of disease. Fifty percent of the participants are
black.
Obligations
Funding History:
Fiscal Year
2006—$6,881,765
Fiscal Years
1984–2005—$71,838,396
Total Funding to Date—$78,720,161
| Current Active
Organizations and Contract Numbers |
- New England Medical Center Hospitals, Inc
Boston, Massachusetts
|
—HC-45204 |
- Wake Forest University Health Sciences
Winston-Salem, North Carolina
|
—HC-45205 |
- University of Alabama at Birmingham
Birmingham, Alabama
|
—HC-48047 |
- University of Minnesota, Twin Cities
Minneapolis, Minnesota
|
—HC-48048 |
- Northwestern University
Chicago,
Illinois
|
—HC-48049 |
- Kaiser Permanente Division of Research
Oakland, California
|
—HC-48050 |
- University of Alabama at Birmingham
Birmingham, Alabama
|
—HC-95095 |
Back to Top
DNA Resequencing and Genotyping, Initiated in Fiscal
Year 2004
The purpose of this program is to obtain rapid,
reliable, and cost-efficient DNA sequencing and genotyping of candidate genomic
regions potentially important in the disease pathways of heart, lung, and blood
diseases and sleep disorders. This information will assist ongoing
investigations of genetic components involved in the causes, variable outcome,
and progression of the diseases and disorders.
Obligations
Funding History:
Fiscal Year
2006—$7,000,000
Fiscal Year
2004-2005—$11,000,000
Total Funding to Date—$18,000,000
| Current Active
Organizations and Contract Numbers |
- Constella Group, Inc.
Bethesda,
Maryland
|
—HV-48193 |
- University of Washington
Seattle,
Washington
|
—HV-48194 |
- Johns Hopkins University
Baltimore,
Maryland
|
—HV-48195 |
- Center for the Advancement of Genetics,
Inc.
Rockville, Maryland
|
—HV-48196 |
Framingham Heart Study
The orginal Framingham Heart Study was designed as a
longitudinal investigation of constitutional and environmental factors
influencing the development of CVD in individuals free of these conditions at
the outset. Of the original 5,209 subjects, about 500 members remain alive. In
1971, the Framingham Offspring Study was initiated to assess familial and
genetic factors associated with CHD. More than 5,000 offspring (and their
spouses) were included. A third-generation cohort consisting of 3,500
grandchildren has been added to permit examination of numerous hypotheses about
the genetic contribution to CVD and CVD risk factors. Additional goals include
identifying new risk factors for cardiovascular, lung, and blood diseases and
developing new imaging tests that can detect very early stages of coronary
atherosclerosis in otherwise healthy adults.
Obligations
Funding History:
Fiscal Year
2006—$10,907,043
Fiscal Years
1983–2005—$62,166,638
Total Funding to Date—$73,073,681
| Current Active
Organization and Contract Number |
- Boston University Medical Center
Boston,
Massachusetts
|
—HC-25195 |
Genetically Triggered Thoracic Aortic Aneurysms and
Other Cardiovascular Conditions (GENTAC): National Registry, Initiated in
Fiscal Year 2006
The purpose of this program is to establish a national
registry to enable investigators to determine the best medical practices to
advance the clinical management of genetic thoracic aortic aneurysms and other
cardiovascular complications associated with connective tissue diseases such as
Marfan Syndrome.
Obligations
Funding History:
Fiscal Year
2006—$1,391,748
Total Funding to Date—$1,391,748
| Current Active
Organization and Contract Number |
- Research Triangle Institute
Research
Triangle Park, North Carolina
|
—HV-68199 |
Hispanic Community Health Study (HCHS), Initiated in
Fiscal Year 2006
The purpose of this study is to identify risk factors
for cardiovascular and lung diseases in Hispanic populations living in the
United States and determine the role of acculturation in their development and
prevalence. The program will support a multicenter, 6.5-year epidemiologic
study comprising approximately 16,000 participants of Hispanic origin (4,000 at
each of 4 sites), aged 18 to 74 years.
Obligations
Funding History:
Fiscal Year
2006—$2,900,000
Total Funding to Date—$2,900,000
| Current Active
Organizations and Contract Numbers |
- University of North Carolina at Chapel Hill
Chapel Hill, North Carolina
|
—HC-65233 |
- University of Miami
Miami, Florida
|
—HC-65234 |
- Albert Einstein College of Medicine
New
York, New York
|
—HC-65235 |
- Northwestern University
Chicago,
Illinois
|
—HC-65236 |
- San Diego State University
San Diego,
California
|
—HC-65237 |
Jackson Heart Study (JHS), Initiated in Fiscal Year
1998
The JHS is a single-site epidemiologic study of CVD in
blacks, similar to established studies in Framingham, Massachusetts, and
Honolulu, Hawaii, with primary goals of identifying risk factors for
development and progression of CVD; enhancing recruitment, cohort retention,
and scientific productivity of the existing Jackson site of the ARIC study;
building research capabilities at minority institutions; developing
partnerships between minority and majority institutions; and expanding minority
investigator participation in large-scale epidemiologic studies.
Obligations
Funding History:
Fiscal Year
2006—$3,535,576
Fiscal Years
1998–2005—$20,219,989
Total Funding to Date—$23,755,565
| Current Active
Organizations and Contract Numbers |
- Jackson State University
Jackson,
Mississippi
|
—HC-95170 |
- Mississippi Medical Center
Jackson,
Mississippi
|
—HC-95171 |
- Tougaloo College
Tougaloo,
Mississippi
|
—HC-95172 |
Back to Top
Multi-Ethnic Study of Atherosclerosis (MESA),
Initiated in Fiscal Year 1999
The purpose of this study is to investigate the
prevalence, correlates, and progression of subclinical CVD, i.e., disease
detected noninvasively before it has produced clinical signs and symptoms, in a
population that is 38 percent white, 28 percent black, 22 percent Hispanic, and
12 percent Asian.
Obligations
Funding History:
Fiscal Year
2006—$8,563,705
Fiscal Years
1999–2005—$54,269,999
Total Funding to Date—$62,833,704
| Current Active
Organizations and Contract Numbers |
- University of Washington
Seattle,
Washington
|
—HC-95159 |
- University of California, Los Angeles
Los
Angeles, California
|
—HC-95160 |
- Columbia University
New York, New
York
|
—HC-95161 |
- Johns Hopkins University
Baltimore,
Maryland
|
—HC-95162 |
- University of Minnesota, Twin
Cities
Minneapolis, Minnesota
|
—HC-95163 |
- Northwestern University
Chicago,
Illinois
|
—HC-95164 |
- Wake Forest University
Winston-Salem,
North Carolina
|
—HC-95165 |
- University of Vermont
Colchester,
Vermont
|
—HC-95166 |
- New England Medical Center
Boston,
Massachusetts
|
—HC-95167 |
- Johns Hopkins University
Baltimore,
Maryland
|
—HC-95168 |
- Harbor-UCLA Research and Education
Institute
Los Angeles, California
|
—HC-95169 |
Pediatric Circulatory Support, Initiated in Fiscal
Year 2004
The purpose of this program is to establish
multidisciplinary teams to develop innovative circulatory assist devices or
other bioengineered systems for infants and children with congenital and
acquired CVD who experience cardiopulmonary failure and circulatory
collapse.
Obligations
Funding History:
Fiscal Year
2006—$5,959,141
Fiscal Year
2004–2005—$8,581,202
Total Funding to Date—$14,540,343
| Current Active
Organizations and Contract Numbers |
- Cleveland Clinic Lerner College of
Medicine
Cleveland, Ohio
|
—HV-48188 |
- Ension, Inc.
Pittsburgh,
Pennsylvania
|
—HV-48189 |
- Jarvik Heart, Inc.
New York, New York
|
—HV-48190 |
- Pennsylvania State University
Hershey,
Pennsylvania
|
—HV-48191 |
- University of Pittsburgh
Pittsburgh,
Pennsylvania
|
—HV-48192 |
Proteomics Initiative, Initiated in Fiscal Year
2002
The purpose of this program is to establish highly
interactive, multidisciplinary centers to enhance and develop innovative
proteomic technologies directed to relevant biologic questions associated with
heart, lung, blood, and sleep health and disease. Scientists will focus on the
cells’ protein machinery directed toward understanding the molecular basis
of the causes and progression of heart, lung, and blood diseases and sleep
disorders and identifying targets for therapeutic interventions.
Obligations
Funding History:
Fiscal Year
2006—$18,740,000
Fiscal Years
2002–2005—$78,893,890
Total Funding to Date—$97,633,890
| Current Active
Organizations and Contract Numbers |
- Boston University
Boston,
Massachusetts
|
—HV-28178 |
- Institute for Systems Biology
Seattle,
Washington
|
—HV-28179 |
- Johns Hopkins University
Baltimore,
Maryland
|
—HV-28180 |
- Medical University of South
Carolina
Charleston, South Carolina
|
—HV-28181 |
- Medical College of Wisconsin
Milwaukee,
Wisconsin
|
—HV-28182 |
- Stanford University
Stanford,
California
|
—HV-28183 |
- University of Texas
Galveston, Texas
|
—HV-28184 |
- University of Texas Southwestern Medical
Center
Dallas, Texas
|
—HV-28185 |
- Yale University
New Haven,
Connecticut
|
—HV-28186 |
- Henry M. Jackson Foundation for the
Advancement of Military Medicine, Inc.
Rockville, Maryland
|
—HV-28187 |
Registry for Mechanical Circulatory Support,
Initiated in Fiscal Year 2005
The purpose of this program is to establish a data and
clinical coordinating center to manage a registry of patients receiving a
mechanical circulatory support device (MCSD) to treat heart failure. The
registry will collect and analyze clinical and laboratory data and tissue
samples from patients who receive MCSDs as destination therapy for end-stage
heart failure at 60 to 70 participating hospitals.
Obligations
Funding History:
Fiscal Year
2006—$1,585,877
Fiscal Year 2005—$1,215,702
Total Funding to Date—$2,801,579
| Current Active
Organization and Contract Number |
- University of Alabama
Birmingham,
Alabama
|
—HV-58198 |
SNP Health Association Resource (SHARE), Initiated in
Fiscal Year 2006
The purpose of this program is to identify genetic
variants associated with heart, lung, and blood diseases and sleep disorders
through application of large-scale, single nucleotide polymorphism (SNP)
genotyping for genome-wide association analyses. In collaboration with the
National Center for Biotechnology Information, the Institute is developing a
public-use data resource to integrate genome-wide genotypic information with
phenotypic information from multiple NHLBI studies.
Obligations
Funding History:
Fiscal Year
2006—$2,000,000
Total Funding to Date—$2,000,000
| Current Active
Organization and Contract Number |
- Affymetrix, Inc.
Santa Clara,
California
|
—HL-64278 |
Back to Top
Translational Behavioral Science Research Consortium,
Initiated in Fiscal Year 2002
The purpose of this program is to establish a
consortium of interdisciplinary basic and applied social scientists to conduct
research related to developing and testing theories from the behavioral or
social sciences concerning cognitive, affective, motivational, developmental,
and other factors and processes underlying human behavior. Acquired knowledge
will be used to develop and test methods to encourage individuals to adopt and
maintain a healthy lifestyle and manage behavioral risk factors for heart,
lung, and blood diseases and sleep disorders.
Obligations
Funding History:
Fiscal Year
2006—$0
Fiscal Years
2002–2005—$20,430,569
Total Funding to Date—$20,430,569
| Current Active
Organizations and Contract Numbers |
- Weill Medical College of Cornell
University
New York, New York
|
—HC-25196 |
- Mount Sinai School of Medicine
New York,
New York
|
—HC-25197 |
Back to Top
Lung Diseases Program
Lung Tissue Research Consortium, Initiated in Fiscal
Year 2004
The purpose of this program is to establish a
consortium for collecting lung tissues and preparing and distributing them for
research. Scientists seek to improve management of lung diseases by increasing
understanding of the pathogenetic mechanisms of lung diseases through molecular
histopathological studies on tissues with and without disease. Primary emphases
are on COPD and idiopathic pulmonary fibrosis.
Obligations
Funding History:
Fiscal Year
2006—$12,598,812
Fiscal Year
2004–2005—$10,499,994
Total Funding to Date—$23,098,806
| Current Active
Organizations and Contract Numbers |
- Mayo Clinic College of Medicine
Rochester,
New York
|
—HR-46158 |
- University of Colorado Health Science
Center
Denver, Colorado
|
—HR-46159 |
- University of Colorado Health Science
Center
Denver, Colorado
|
—HR-46160 |
- Mayo Clinic College of Medicine
Rochester,
New York
|
—HR-46161 |
- University of Michigan
Ann Arbor,
Michigan
|
—HR-46162 |
- University of Pittsburgh
Pittsburgh,
Pennsylvania
|
—HR-46163 |
- Clinical Trials and Survey
Corporation
Baltimore, Maryland
|
—HR-46164 |
Tuberculosis Curriculum Coordinating Center,
Initiated in Fiscal Year 2003
The purpose of this program is to establish a
consortium of five Tuberculosis Curriculum Centers to strengthen and increase
access to the best ongoing educational and training opportunities in TB for
medical, nursing, and allied health schools, especially those that provide
primary care to communities where TB is endemic and the population is at high
risk.
Obligations
Funding History:
Fiscal Year
2006—$1,125,000
Fiscal Years
2003–2005—$3,750,000
Total Funding to Date—$4,875,000
| Current Active
Organizations and Contract Numbers |
- University of California, San Diego
La
Jolla, California
|
—HR-36157 |
Back to Top
Blood Diseases and Resources
Program
Iron Overload and Hereditary Hemochromatosis,
Initiated in Fiscal Year 2000
The purpose of this program is to determine the
prevalence; genetic and environmental determinants; and potential clinical,
personal, and societal impact of iron overload and hereditary hemochromatosis
in a multicenter, multiethnic, primary care-based sample of 100,000 adults.
Information derived from the study will be used to determine the feasibility
and potential individual and public health benefits and risks of primary
care-based screening and intervention for iron overload and hereditary
hemochromatosis.
Obligations
Funding History:
Fiscal Year
2006—$469,227
Fiscal Year
2000–2005—$25,979,773
Total Funding to Date—$26,449,000
| Current Active
Organizations and Contract Numbers |
- University of Minnesota
Minneapolis,
Minnesota
|
—HC-05185 |
- University of Alabama at
Birmingham
Birmingham, Alabama
|
—HC-05188 |
- London Health Science
London, Canada
|
—HC-05191 |
- Wake Forest University
Winston-Salem,
North Carolina
|
—HC-05192 |
Maintenance of Animals for Hepatitis or AIDS
Research, Initiated in Fiscal Year 1992
The purpose of this program is to maintain an NHLBI
chimpanzee colony that can be used in experiments with transfusion-transmitted
infectious agents (hepatitis C virus (HCB), hepatitis B virus (HBV), and HIV)
for which the chimpanzee is the only known animal model.
Obligations
Funding History:
Fiscal Year
2006—$160,474
Fiscal Year
1992–2005—$8,805,530
Total Funding to Date—$8,966,004
| Current Active
Organizations and Contract Numbers |
- Southwest Foundation for Biomedical
Research
San Antonio, Texas
|
—HB-27091 |
Maintenance of NHLBI Biological Specimen Repository,
Initiated in Fiscal Year 1998
The purpose of this project is to establish an NHLBI
Biological Specimen Repository for blood specimens from Institute-supported
research. The Repository monitors storage, labeling, and testing of the
specimens, as well as administers safe shipment of precise sample aliquots to
approved investigators for future studies.
Obligations
Funding History:
Fiscal Year
2006—$1,031,572
Fiscal Years
1998–2005—$6,594,342
Total Funding to Date—$7,625,914
| Current Active
Organizations and Contract Numbers |
- BBI-Biotech Research Laboratories,
Inc.
Gaithersburg, Maryland
|
—HB-87144 |
Back to Top
Production of Recombinant B19 Parvovirus Capsids,
Initiated in Fiscal Year 1998
The purpose of this program is to manufacture
clinical-grade recombinant protein Parvovirus B19 vaccine for use in a Phase
I/II clinical trial to be conducted by the NHLBI.
Obligations
Funding History:
Fiscal Year
2006—$666,950
Fiscal Year
1998–2005—$2,483,750
Total Funding to Date—$3,150,700
| Current Active
Organizations and Contract Numbers |
- Viral Antigens, Inc.
Memphis,
Tennessee
|
—HB-37167 |
Retrovirus Epidemiology Donor Study (REDS), Initiated
in Fiscal Year 1989
The purpose of this study is to conduct epidemiologic,
laboratory, and survey research on volunteer blood donors within the United
States to ensure the safety and availability of the blood supply. The studies
will examine the risks of transfusion-transmissible infections, their trends,
and ways to reduce infection risks; HIV, HTLV, HCV, and HBV test screening
methodologies; donor characteristics, behaviors, and donation return patterns
of U.S. blood donors; and the effectiveness and safety of various strategies to
increase the U.S. blood supply.
Obligations
Funding History:
Fiscal Year
2006—$8,955,049
Fiscal Years
1989–2005—$86,865,125
Total Funding to Date—$95,820,174
| Current Active
Organizations and Contract Numbers |
- Blood Center of Southeastern
Wisconsin
Milwaukee, Wisconsin
|
—HB-47168 |
- Emory University
Atlanta, Georgia
|
—HB-47170 |
- Institute for Transfusion
Medicine
Pittsburgh, Pennsylvania
|
—HB-47172 |
- Westat, Inc.
Rockville, Maryland
|
—HB-47175 |
- Blood System Research, Inc.
San Francisco,
California
|
—HB-57181 |
Sickle Cell Disease Health-Related Quality of Life
Questionnaire, Initiated in Fiscal Year 2005
The purpose of this project is to develop a
psychometrically sound and clinically useful health-related quality-of-life
instrument and related materials for use in sickle cell clinical trials and
outcomes research among adults with SCD, and to assist researchers who are
early users of the instrument and materials.
Obligations
Funding History:
Fiscal Year
2006—$708,000
Fiscal Year 2005—$584,008
Total Funding To Date—$1,292,008
| Current Active
Organizations and Contract Numbers |
- American Institutes for Research Health
Program
Silver Spring, Maryland
|
—HL-54264 |
Somatic Cell Therapy Processing Facilities, Initiated
in Fiscal Year 2003
This program is designed to develop novel somatic
cellular therapies in areas ranging from basic science through animal studies
to proof-of-principle and eventually human trials for heart, lung, and blood
diseases and sleep disorders. The goal is to provide rapid, safe translation of
basic research ideas into clinical practice.
Obligations
Funding History:
Fiscal Year
2006—$6,139,526
Fiscal Years
2003–2005—$15,576,209
Total Funding to Date—$21,715,735
| Current Active
Organizations and Contract Numbers |
- Baylor College of Medicine
Houston,
Texas
|
—HB-37163 |
- University of Minnesota, Twin
Cities
Minneapolis, Minnesota
|
—HB-37164 |
- University of Pittsburgh
Pittsburgh,
Pennsylvania
|
—HB-37165 |
- The EMMES Corporation
Rockville,
Maryland
|
—HB-37166 |
Back to Top
« Factbook Table of
Contents |