PLCO Background Information
The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial is a large population-based randomized trial evaluating screening programs for these cancers. The primary goal of this long-term trial of the National Cancer Institute's (NCI) Division of Cancer Prevention (DCP) is to determine the effects of screening on cancer-related mortality and on secondary endpoints.
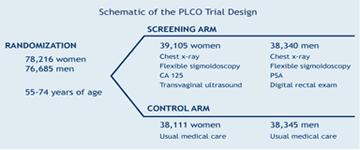
Ten screening centers located across the United States enrolled approximately 77,500 men and 77,500 women, ages 55 to 74, and randomized them to an intervention arm, which received trial screening, or control arm, which received standard care. Participants included in the intervention arm of the trial received screening for the PLCO cancers during their first 6 years of participation in the trial, and follow-up continued for at least 7 additional years. Participants in the control arm were followed for 13 years after enrollment, but did not receive the screening examination. The ten PLCO Screening Centers began enrolling participants in 1993 and completed enrollment in 2001. Trial participants are uncompensated volunteers recruited from the general population in the geographic area of each of the screening centers. Eligibility requirements included age (between 55 and 74 at enrollment), and no previous history of any PLCO cancer.
Trial data include screening data, baseline and follow-up health and risk data, dietary data, health status, and the collection of blood samples that will support molecular, behavioral and risk studies in early detection and etiology.
 [ Click to enlarge ]
[ Click to enlarge ]
Back to top