News & Events
FDA User Fee Agreements: Strengthening FDA and the Medical Products Industry for the Benefit of Patients (JW)
Statement of
Janet Woodcock, M.D.
Director, Center for Drug Evaluation and Research
Food and Drug Administration
Department of Health and Human Services
Before the
Committee on Health, Education, Labor and Pensions
United States Senate
March 29, 2012
INTRODUCTION
Mr. Chairman and Members of the Subcommittee, I am Dr. Janet Woodcock, Director of the Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration (FDA or the Agency), which is part of the Department of Health and Human Services (HHS). Thank you for the opportunity to be here today to discuss the fifth reauthorization of the Prescription Drug User Fee Act (PDUFA) [1], also referred to as PDUFA V, as well as the negotiated recommendations for a generic drug user fee program and a biosimilar user fee program.
Background on PDUFA
FDA considers the timely review of the safety and effectiveness of New Drug Applications (NDA) and Biologics License Applications (BLA) to be central to the Agency’s mission to protect and promote the public health. Prior to enactment of PDUFA in 1992, FDA's review process was understaffed, unpredictable, and slow. FDA lacked sufficient staff to perform timely reviews, or develop procedures and standards to make the process more rigorous, consistent, and predictable. Access to new medicines for U.S. patients lagged behind other countries. As a result of concerns expressed by both industry and patients, Congress enacted PDUFA, which provided the added funds through user fees that enabled FDA to hire additional reviewers and support staff and upgrade its information technology systems. At the same time, FDA committed to complete reviews in a predictable time frame. These changes revolutionized the drug approval process in the United States and enabled FDA to speed the application review process for new drugs, without compromising the Agency’s high standards for demonstration of safety, efficacy, and quality of new drugs prior to approval.
Three fees are collected under PDUFA: application fees, establishment fees, and product fees. An application fee must be submitted when certain NDAs or BLAs are submitted. Product and establishment fees are due annually. The total revenue amounts derived from each of the categories—application fees, establishment fees, and product fees—are set by the statute for each fiscal year (FY). PDUFA permits waivers under certain circumstances, including a waiver of the application fee for small businesses and orphan drugs.
Of the total $931,845,581 obligated in support of the process for the review of human drug applications in FY 2010, PDUFA fees funded 62 percent, with the remainder funded through appropriations.
PDUFA Achievements
PDUFA has produced significant benefits for public health, providing patients faster access to over 1,500 new drugs and biologics, since enactment in 1992, including treatments for cancer, infectious diseases, neurological and psychiatric disorders, and cardiovascular diseases. In FY 2011, FDA approved 35 new, groundbreaking medicines, including two treatments for hepatitis C, a drug for late-stage prostate cancer, the first drug for Hodgkin’s lymphoma in 30 years, and the first drug for lupus in 50 years. This was the second highest number of annual approvals in the past 10 years, surpassed only by 2009. Of the 35 innovative drugs approved in FY 2011, 34 met their PDUFA target dates for review.
Substantially Reduced Review Times
PDUFA provides FDA with a source of stable, consistent funding that has made possible our efforts to focus on promoting innovative therapies and help bring to market critical products for patients.
According to researchers at the Tufts Center for the Study of Drug Development, the time required for the FDA approval phase of new drug development (i.e., time from submission until approval) has been cut since the enactment of PDUFA in 1992, from an average of 2 years for the approval phase at the start of PDUFA to an average of 1.1 years more recently.[2]
FDA aims to review priority drugs more quickly, in six months vs. 10 months for standard drugs. Priority drugs are generally targeted at severe illnesses with few or no available therapeutic options. FDA reviewers give these drugs priority attention throughout development, working with sponsors to determine the most efficient way to collect the data needed to provide evidence of safety and effectiveness.
Reversal of the “Drug Lag”
Importantly, PDUFA has led to the reversal of the drug lag that prompted its creation. Since the enactment of PDUFA, FDA has steadily increased the speed of Americans’ access to important new drugs compared to the European Union (EU) and the world as a whole. Of the 35 innovative drugs approved in FY 2011, 24 (almost 70 percent) were approved by FDA before any other regulatory agency in the world, including the European Medicines Agency. Of 57 novel drugs approved by both FDA and the EU between 2006 and 2010, 43 (75 percent) were approved first in the United States.
Figure 1 below shows that since the late 1990s, the United States has regularly led the world in the first introduction of new active drug substances.[3] Preliminary data show that in 2011, over half of all new active drug substances were first launched in the United States.[4]
Figure 1. U.S. Share of New Active Substances (NAS) First Launched on the World Market
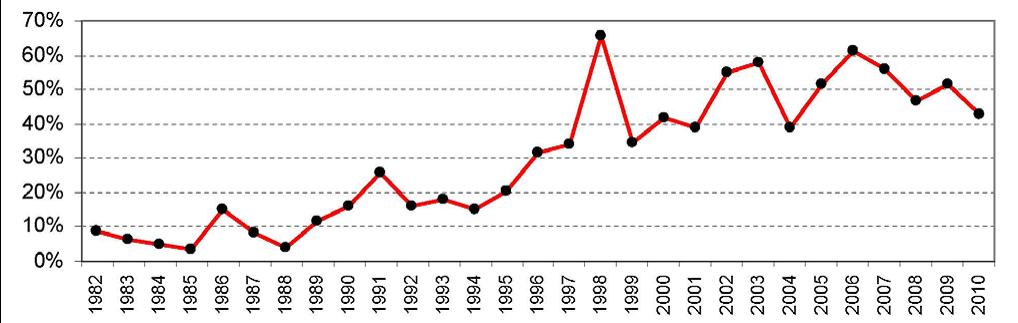
Year of NAS First Launch Worldwide
In recent years, FDA’s drug review times also have been, on average, significantly faster than those in the EU. It is difficult to compare length of approvals for FY 2011, because many of the drugs approved in the United States have not yet been approved in the EU. A comparison of drugs approved in the United States and the EU between 2006 and 2010 is illustrative, however. For priority drugs approved between 2006 and 2010, FDA’s median time to approval was six months (183 days), more than twice as fast as the EU, which took a median time of 13.2 months (403 days). For standard drug reviews, FDA’s median time to approval was 13 months (396 days), 53 days faster than the EU time of 14.7 months (449 days).
A recent article in the journal Health Affairs also compared cancer drugs approved in the United States and EU from 2003 through 2010. Thirty-five cancer drugs were approved by the United States or the EU from October 2003 through December 2010. Of those, FDA approved 32—in an average time of 8.6 months (261 days). The EU approved only 26 of these products, and its average time was 12.2 months (373 days). This difference in approval times is not due to safety issues with these products. All 23 cancer drugs approved by both agencies during this period were approved first in the United States.
Speeding Access to New Therapies
PDUFA funds help support a number of existing FDA programs to expedite the approval of certain promising investigational drugs, and also to make them available to the very ill before they have been approved for marketing, without unduly jeopardizing patient safety.
The most important of these programs are Accelerated Approval, Fast Track, and Priority Review. In 1992, FDA instituted the Accelerated Approval process, which allows earlier approval of drugs that treat serious or life-threatening diseases and that fill an unmet medical need based on a surrogate endpoint that is reasonably likely to predict clinical benefit but is not fully validated to do so, or, in some cases, an effect on a clinical endpoint other than survival or irreversible morbidity. A surrogate endpoint is a marker—a laboratory measurement, or physical sign—that is used in clinical trials as an indirect or substitute measurement for a clinically meaningful outcome, such as survival or symptom improvement. For example, viral load is a surrogate endpoint for approval of drugs for the treatment of HIV/AIDS. The use of a surrogate endpoint can considerably shorten the time to approval, allowing more rapid patient access to promising new treatments for serious or life-threatening diseases. Accelerated Approval is given on the condition that sponsors conduct post-marketing clinical trials to verify the anticipated clinical benefit.
Over 80 new products have been approved under Accelerated Approval since the program was established, including 29 drugs to treat cancer, 32 to treat HIV, and 20 to treat other conditions such as pulmonary arterial hypertension, Fabry disease, and transfusion-dependent anemia. Three of the 30 new molecular entities (NMEs) and new BLAs approved in 2011 in CDER were approved under Accelerated Approval. Corifact, the first treatment approved for a rare blood-clotting disorder, also was approved under Accelerated Approval in FDA’s Center for Biologics Evaluation and Research (CBER) on February 17, 2011.
Fast Track is a process designed to facilitate the development, and expedite the review, of drugs to treat serious or life-threatening diseases that will fill an unmet medical need. Once a drug receives Fast-Track designation, early and frequent communications between FDA and a drug company are encouraged throughout the entire drug development and review process. The frequency of communications ensures that questions and issues are resolved quickly, often leading to earlier drug approval and access by patients. For example, Zelboraf (vemurafenib) was given a Fast-Track designation because it had the potential to improve overall survival in patients with melanoma, the most dangerous type of skin cancer. Because of convincing early findings with this drug, FDA scientists worked proactively with the sponsor during drug testing to encourage early submission of the application. FDA approved Zelboraf in 2011 to treat patients with late-stage (metastatic) or unresectable (cannot be removed by surgery) melanoma.
In 1992, under PDUFA, FDA agreed to specific goals for improving drug review times and created a two-tiered system of review times—Priority Review and Standard Review. FDA aims to review priority drugs more quickly, in six months versus 10 months for standard drugs. Priority review designation is given to drugs that offer major advances in treatment, or provide a treatment where no adequate therapy exists, while Standard Review is applied to drugs that offer at most only minor improvement over existing marketed therapies. FDA reviewers give Priority Review drugs priority attention throughout development, working with sponsors to determine the most efficient way to collect the data needed to provide evidence of safety and effectiveness. For example, on January 31, 2012, FDA approved Kalydeco (ivacaftor) to treat patients age 6 or older with Cystic Fibrosis (CF) and who have a specific genetic defect (G551D mutation), after a Priority Review. CF occurs in approximately 30,000 children and adults in the United States. The G551D mutation occurs in approximately 4 percent of patients with CF, totaling approximately 1,200 patients in the United States. CF is a serious inherited disease that affects the lungs and other organs in the body, leading to breathing and digestive problems, trouble gaining weight, and other problems. There is no cure for CF, and despite progress in the treatment of the disease, most patients with CF have shortened life spans and do not live beyond their mid-30’s. After the results of studies of ivacaftor showed a significant benefit to patients with CF with the G551D mutation, ivacaftor was reviewed and approved by FDA in approximately three months—half of the Priority Review period. Ivacaftor is the first medicine that targets the underlying cause of CF; to date, therapy has aimed at treating symptoms or complications of the disease.
FDA also recognizes circumstances in which there is public health value in making products available prior to marketing approval. A promising but not yet fully evaluated treatment may sometimes represent the best choice for individuals with serious or life-threatening diseases who lack a satisfactory therapy.
FDA allows for access to investigational products through multiple mechanisms. Clinical trials are the best mechanism for a patient to receive an investigational drug, because they provide a range of patient protections and benefits and they maximize the gathering of useful information about the product, which benefits the entire patient population. However, there are times when an individual cannot enroll in a clinical trial. In some cases, the patient may gain access to an investigational therapy through one of the alternative mechanisms, and FDA’s Office of Special Health Issues assists patients and their doctors in this endeavor.
We are committed to using these programs to speed therapies to patients while upholding our high standards of safety and efficacy. Balancing these two objectives requires that we continue to evaluate our use of the tools available to us and consider whether additional tools would be helpful. We are eager to work with Congress in this area, and we note that several of the enhancements proposed for PDUFA V are aimed at expediting the availability of new therapies and providing FDA the scientific understanding necessary to modernize and streamline our regulatory process.
Providing Guidance to Industry
Increased resources provided by user fees have enabled FDA to provide a large body of technical guidance to industry that clarified the drug development pathway for many diseases, and to meet with companies during drug development to provide critical advice on specific development programs. In the past five years alone, FDA has held over 7,000 formal meetings with drug sponsors within a short time after a sponsor’s request. Innovations in drug development are being advanced by many new emerging companies as well as more established ones, and new sponsors may need, and often seek, more regulatory guidance during development. In FY 2009 through FY 2011, more than half of the meetings FDA held during drug development were with companies that had no approved product on the U.S. market.
Weighing Benefit and Risk
It should be noted that FDA assesses the benefit-risk of new drugs on a case-by-case basis, considering the degree of unmet medical need and the severity and morbidity of the condition the drug is intended to treat. This approach has been critical to increasing patient access to new drugs for cancer and rare and other serious diseases, where existing therapies have been few and limited in their effectiveness. Some of these products have serious side effects but they were approved because the benefit outweighed the risk. For example, in March of last year, FDA approved Yervoy (ipilimumab) for the treatment of unresectable or metastatic melanoma. Yervoy also poses a risk of serious side effects in 12.9 percent of patients treated, including severe to fatal autoimmune reactions. However, FDA decided that the benefits of Yervoy outweighed its risks, especially considering that no other melanoma treatment has been shown to prolong a patient’s life.
As discussed in more detail below, PDUFA V will enable FDA to develop an enhanced, structured approach to benefit-risk assessments that accurately and concisely describes the benefit and risk considerations in the Agency’s drug regulatory decision-making.
Challenges for the Current Drug Program
Although we can report many important successes with the current program, new challenges have also emerged that offer an opportunity for further enhancement. While new authorities from the Food and Drug Administration Amendments Act of 2007 (FDAAA) have strengthened drug safety, they have put strains on FDA’s ability to meet premarket review performance goals and address post-market review activities. In addition, there has been a significant increase in the number of foreign sites included in clinical trials to test drug safety and effectiveness, and an increase in the number of foreign facilities used in manufacturing new drugs for the U.S. market. While foreign sites can play an important role in enabling access to new drugs, the need to travel much farther to conduct pre-approval inspections for clinical trials and manufacturing sites overseas has created additional challenges for completion of FDA’s review within the existing PDUFA review performance goals, while at the same time trying to communicate
with sponsors to see if identified issues can be resolved before the review performance goal date.
Despite these challenges, FDA has maintained strong performance in meeting the PDUFA application review goals, with the exception of a dip in FY 2008-09, when staff resources were shifted within the discretion afforded FDA to ensure timely implementation of all the new FDAAA provisions that affected activities in the new drug review process. Recent performance data show that FDA has returned to meeting or exceeding goals for review of marketing applications under PDUFA. This is shown in Figure 3.
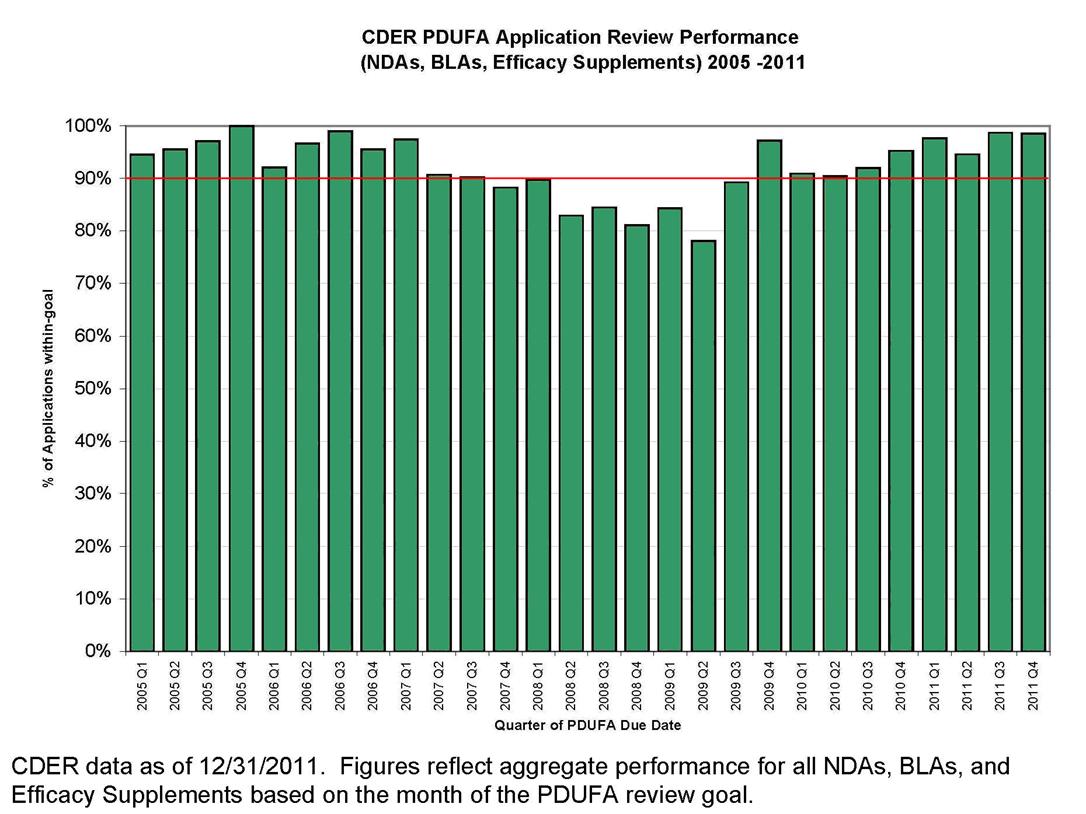
However, FDA wants to meet not only the letter, but also the spirit of the PDUFA program. That is, we want to speed patient access to drugs shown to be safe and effective for the indicated uses while also meeting our PDUFA goals.
The NDA/BLA approval phase of drug development is reported to have the highest success rate of any phase of drug development. That is, the percentage of drugs that fail after the sponsor submits an NDA/BLA to FDA is less than the percentages that fail in preclinical development and in each phase of clinical development. At the same time, it is critical to our public health mission that we work with industry and other stakeholders to take steps to reduce uncertainty and increase the success of all phases of drug development. We must leverage advances in science and technology to make sure that we have the knowledge and tools we need to rapidly and meaningfully evaluate medical products. The science of developing new tools, standards, and approaches to assess the safety, efficacy, quality, and performance of FDA-regulated products—known as regulatory science—is about more than just speeding drug development prior to the point at which FDA receives an application for review and approval. It also gives us the scientific tools to modernize and streamline our regulatory process. With so much at stake for public health, FDA has made advances in regulatory science a top priority. The Agency is both supporting mission-critical science at FDA and exploring a range of new partnerships with the National Institutes of Health (NIH) and academic institutions to develop the science needed to maximize advances in biomedical research and bring the development and assessment of promising new therapies and devices into the 21st century. With this effort, FDA is poised to support a wave of innovation to transform medicine and save lives.
For example, FDA is working to improve the science behind certain clinical trial designs. Recent advances in two clinical trial designs—called non-inferiority and adaptive designs—have required FDA to conduct more complex reviews of clinical trial protocols and new marketing applications. Improving the scientific bases of these trial designs should add efficiency to the drug review process, encourage the development of novel products, and speed new therapies to patients.
FDA also has taken steps to help facilitate the development and approval of safe and effective drugs for Americans with rare diseases. Therapies for rare diseases—those affecting fewer than 200,000 people in the United States—represent the most rapidly expanding area of drug development. Although each disease affects a relatively small population, collectively, rare diseases affect about 25 million Americans. Approximately one-third of the NMEs and new biological products approved in the last five years have been drugs for rare diseases. Because of the small numbers of patients who suffer from each disease, FDA often allows non-traditional approaches to establishing safety and effectiveness. For example, FDA approved Voraxaze (glucarpidase) in January 2012 to treat patients with toxic methotrexate levels in their blood due to kidney failure, which affects a small population of patients each year. Methotrexate is a commonly used cancer chemotherapy drug normally eliminated from the body by the kidneys.
Patients receiving high doses of methotrexate may develop kidney failure. Voraxaze was approved based on data in 22 patients from a single clinical trial, which showed decreased levels of methotrexate in the blood. Prior to the approval of Voraxaze, there were no effective therapies for the treatment of toxic methotrexate levels in patients with renal failure.
PDUFA Reauthorization
In PDUFA IV, Congress directed FDA to take additional steps to ensure that public stakeholders, including consumer, patient, and health care professional organizations, would have adequate opportunity to provide input to the reauthorization and any program enhancements for PDUFA V. Congress directed the Agency to hold an initial public meeting and then to meet with public stakeholders periodically, while conducting negotiations with industry to hear their views on the reauthorization and their suggestions for changes to the PDUFA performance goals. PDUFA IV also required that minutes from negotiation sessions held with industry be made public.
Based on a public meeting held in April 2010, input from a public docket, and the Agency’s own internal analyses of program challenge areas, FDA developed a set of potential proposed enhancements for PDUFA V and in July 2010, began negotiations with industry and parallel discussions with public stakeholders. These discussions concluded in May 2011 and we held a public meeting on October 24, 2011, where we solicited comments on the proposed recommendations. We also opened a public docket for comments. We considered these comments, and on January 13, 2012, we transmitted the final recommendations to Congress.
We are very pleased to report that the enhancements for PDUFA V address many of the top priorities identified by public stakeholders, the top concerns identified by industry, and the most important challenges identified within FDA. I will briefly summarize these enhancements.
A. Review Program for New Drug Applications, New Molecular Entities, and Original Biologics License Applications
FDA’s existing review performance goals for priority and standard applications—six and 10 months respectively—were established in 1997. Since that time, additional requirements in the drug review process have made those goals increasingly challenging to meet, particularly for more complex applications like new molecular entity (NME) NDAs and original BLAs. FDA also recognizes that increasing communication between the Agency and sponsors during the application review has the potential to increase efficiency in the review process.
To address the desire for increased communication and greater efficiency in the review process, we agreed to an enhancement to FDA’s review program for NME NDAs and original BLAs in PDUFA V. This program includes pre-submission meetings, mid-cycle communications, and late-cycle meetings between FDA and sponsors for these applications. To accommodate this increased interaction during regulatory review, as agreed to with industry, FDA’s review clock would begin after the 60-day administrative filing review period for this subset of applications. The impact of these modifications on the efficiency of drug review for this subset of applications will be assessed during PDUFA V.
B. Enhancing Regulatory Science and Expediting Drug Development
The following five enhancements focus on regulatory science and expediting drug development.
1. Promoting Innovation Through Enhanced Communication Between FDA and Sponsors During Drug Development
FDA recognizes that timely interactive communications with sponsors can help foster efficient and effective drug development. In some cases, a sponsor’s questions may be complex enough to require a formal meeting with FDA, but in other instances, a question may be relatively straightforward such that a response can be provided more quickly. However, our review staff’s workload and other competing public health priorities can make it challenging to develop an Agency response to matters outside of the formal meeting process.
This enhancement involves a dedicated drug development communication and training staff, focused on improving communications between FDA and sponsors during development. This staff will be responsible for identifying best practices for communications between the Agency and sponsors, training review staff, and disseminating best practices through published guidance.
2. Methods for Meta-analysis
A meta-analysis typically attempts to combine the data or findings from multiple completed studies to explore drug benefits and risks and, in some cases, uncover what might be a potential safety signal in a premarket or post-market context. However, there is no consensus on best practices in conducting a meta-analysis. With the growing availability of clinical trial data, an increasing number of meta-analyses are being conducted based on varying sets of data and assumptions. If such studies conducted outside FDA find a potential safety signal, FDA will work to try to confirm—or correct—the information about a potential harm. To do this, FDA must work quickly to conduct its own meta-analyses to include publicly available data and the raw clinical trial data submitted by drug sponsors that would typically not be available to outside researchers. This is resource-intensive work and often exceeds the Agency’s on-board scientific and computational capacity, causing delays in FDA findings that prolong public uncertainty.
PDUFA V enhancements include the development of a dedicated staff to evaluate best practices and limitations in meta-analysis methods. Through a rigorous public comment process, FDA would develop guidance on best practices and the Agency’s approach to meta-analysis in regulatory review and decision-making.
3. Biomarkers and Pharmacogenomics
Pharmacogenomics and the application of qualified biomarkers have the potential to decrease drug development time by helping to demonstrate benefits, establish unmet medical needs, and identify patients who are predisposed to adverse events. FDA provides regulatory advice on the use of biomarkers to facilitate the assessment of human safety in early phase clinical studies, to support claims of efficacy, and to establish the optimal dose selection for pivotal efficacy studies. This is an area of new science where the Agency has seen a marked increase in sponsor submissions to FDA. In the 2008-2010 period, the Agency experienced a nearly four-fold increase in this type of review work.
PDUFA V enhancements include augmenting the Agency’s clinical, clinical pharmacology, and statistical capacity to adequately address submissions that propose to utilize biomarkers or pharmacogenomic markers. The Agency would also hold a public meeting to discuss potential strategies to facilitate scientific exchanges on biomarker issues between FDA and drug manufacturers.
4. Use of Patient-reported Outcomes
Assessments of study endpoints known as patient-reported outcomes (PROs) are increasingly an important part of successful drug development. PROs measure treatment benefit or risk in medical product clinical trials from the patients’ point of view. They are critical in understanding drug benefits and harm from the patients’ perspective. However, PROs require rigorous evaluation and statistical design and analysis to ensure reliability to support claims of clinical benefit. Early consultation between FDA and drug sponsors can ensure that endpoints are well-defined and reliable. However, the Agency does not have the capacity to meet the current demand from industry.
PDUFA V enhancements include an initiative to improve FDA’s clinical and statistical capacity to address submissions involving PROs and other endpoint assessment tools, including providing consultation during the early stages of drug development. In addition, FDA will convene a public meeting to discuss standards for PRO qualification, new theories in endpoint measurement, and the implications for multi-national trials.
5. Development of Drugs for Rare Diseases
FDA’s oversight of rare disease drug development is complex and resource intensive. Rare diseases are a highly diverse collection of disorders, their natural histories are often not well-described, only small population sizes are often available for study, and they do not usually have well-defined outcome measures. This makes the design, execution, and interpretation of clinical trials for rare diseases difficult and time consuming, requiring frequent interaction between FDA and drug sponsors. If recent trends in orphan designations are any indication, FDA can expect an increase in investigational activity and marketing applications for orphan products in the future.
Another PDUFA V enhancement includes FDA facilitation of rare disease drug development by issuing relevant guidance, increasing the Agency’s outreach efforts to the rare disease patient community, and providing specialized training in rare disease drug development for sponsors and FDA staff.
C. Enhancing Benefit-Risk Assessment
FDA has been developing an enhanced, structured approach to benefit-risk assessments that accurately and concisely describes the benefit and risk considerations in the Agency’s drug regulatory decision-making. Part of FDA’s decision-making lies in thinking about the context of the decision—an understanding of the condition treated and the unmet medical need. Patients who live with a disease have a direct stake in the outcome of drug review. The FDA drug review process could benefit from a more systematic and expansive approach to obtaining the patient perspective on disease severity and the potential gaps or limitations in available treatments in a therapeutic area.
PDUFA V enhancements include expanded implementation of FDA’s benefit-risk framework in the drug review process, including holding public workshops to discuss the application of frameworks for considering benefits and risks that are most appropriate for the regulatory setting. FDA would also conduct a series of public meetings between its review divisions and the relevant patient advocacy communities to review the medical products available for specific indications or disease states that will be chosen through a public process.
D. Enhancement and Modernization of the FDA Drug Safety System
The enhancements for PDUFA V include two post-market, safety-focused initiatives.
1. Standardizing Risk Evaluation and Mitigation Strategies
FDAAA gave FDA authority to require a Risk Evaluation and Mitigation Strategy (REMS) when FDA finds that a REMS is necessary to ensure that the benefits of a drug outweigh its risks. Some REMS are more restrictive types of risk management programs that include elements to ensure safe use (ETASU). These programs can require such tools as prescriber training or certification, pharmacy training or certification, dispensing in certain health care settings, documentation of safe use conditions, required patient monitoring, or patient registries. ETASU REMS can be challenging to implement and evaluate, involving cooperation of all segments of the health care system. Our experience with REMS to date suggests that the development of multiple individual programs has the potential to create burdens on the health care system and, in some cases, could limit appropriate patient access to important therapies.
PDUFA V enhancements initiate a public process to explore strategies and initiate projects to standardize REMS with the goal of reducing burden on practitioners, patients, and others in the health care setting. Additionally, FDA will conduct public workshops and develop guidance on methods for assessing the effectiveness of REMS and the impact on patient access and burden on the health care system.
2. Using the Sentinel Initiative to Evaluate Drug Safety Issues
FDA’s Sentinel Initiative is a long-term program designed to build and implement a national electronic system for monitoring the safety of FDA-approved medical products. FDAAA required FDA to collaborate with federal, academic, and private entities to develop methods to obtain access to disparate data sources and validated means to link and analyze safety data to monitor the safety of drugs after they reach the market, an activity also known as “active post-market drug safety surveillance.” FDA will use user fee funds to conduct a series of activities to determine the feasibility of using Sentinel to evaluate drug safety issues that may require regulatory action, e.g., labeling changes, post-marketing requirements, or post-marketing commitments. This may shorten the time it takes to better understand new or emerging drug safety issues. PDUFA V enhancements will enable FDA to initiate a series of projects to establish the use of active post-market drug safety surveillance in evaluating post-market safety signals in population-based databases. By leveraging public and private health care data sources to quickly evaluate drug safety issues, this work may reduce the Agency’s reliance on required post-marketing studies and clinical trials.
E. Required Electronic Submissions and Standardization of Electronic Application Data
The predictability of the FDA review process relies heavily on the quality of sponsor submissions. The Agency currently receives submissions of original applications and supplements in formats ranging from paper-only to electronic-only, as well as hybrids of the two media. The variability and unpredictability of submitted formats and clinical data layout present major obstacles to conducting a timely, efficient, and rigorous review within current
PDUFA goal time frames. A lack of standardized data also limits FDA’s ability to transition to more standardized approaches to benefit-risk assessment and impedes conduct of safety analyses that inform FDA decisions related to REMS and other post-marketing requirements. PDUFA V enhancements include a phased-in requirement for standardized, fully electronic submissions during PDUFA V for all marketing and investigational applications. Through partnership with open standards-development organizations, the Agency would also conduct a public process to develop standardized terminology for clinical and non-clinical data submitted in marketing and investigational applications.
F. User Fee Increase for PDUFA V
The cost of the agreed upon PDUFA V enhancements translates to an overall increase in fees of approximately 6 percent.
G. PDUFA V Enhancements for a Modified Inflation Adjuster and Additional Evaluations of the Workload Adjuster
In calculating user fees for each new fiscal year, FDA adjusts the base revenue amount by inflation and workload as specified in the statute. PDUFA V enhancements include a modification to the inflation adjuster to accurately account for changes in its costs related to payroll compensation and benefits as well as changes in non-payroll costs. In addition, FDA will continue evaluating the workload adjuster that was developed during the PDUFA IV negotiations to ensure that it continues to adequately capture changes in FDA’s workload.
Generic Drug User Fees
As a result of the Drug Price Competition and Patent Term Restoration Act of 1984, commonly known as Hatch-Waxman Amendments passed by Congress more than a quarter of a century ago, America’s generic drug industry has been developing, manufacturing, and marketing—and FDA has been reviewing and approving—lower-cost versions of brand-name drugs. This legislation and the industry it fostered has been a true public health success. Last year, approximately 78 percent of the more than 3 billion new and refilled prescriptions dispensed in the United States were filled with generics. In the last decade alone, generic drugs have provided more than $931 billion in savings to the nation’s health care system.[5]
This success, however, also has come to represent a significant regulatory challenge, and delays in approvals of generic drugs have emerged as a major concern for the generics industry, FDA, consumers, and payers alike. Unlike the brand manufacturers who pay fees under PDUFA, the generic industry does not pay a user fee to support FDA activities related to its applications. Over the last several years, the time it takes for FDA to approve a generic drug has nearly doubled as FDA’s resources have not kept pace with an increasing number of Abbreviated New Drug Applications (ANDA) and other submissions related to generic drugs. The number of generic drug submissions sent annually to FDA has grown rapidly, reaching another record high this year, including nearly 1,000 ANDAs. Drug Master Files [6] have grown at a comparable pace and have reached similar heights. The current backlog of applications pending review is estimated to be over 2,500. The current median time to approval is approximately 31 months, though it should be noted that this includes time the application is back with the sponsor to answer any questions FDA may have about the application.
The regulatory challenge of ensuring safe, high-quality generic drugs includes inspecting manufacturing facilities, where the challenge is not just one of numbers but also of geography. To keep pace with the growth of the generic drug industry, FDA has had to conduct more inspections as the number of facilities supporting those applications has also increased, with the greatest increase coming from foreign facilities. Currently, the number of foreign Finished Dosage Form (FDF) [7] manufacturers exceeds the number found in the United States. The generic industry is also experiencing significant growth in India and China, a trend expected to continue. Foreign inspections represent a significant challenge and require significant resources.
The generic drug user fee agreement is designed to address the regulatory challenges mentioned above in an affordable manner. The annual fee total proposed represents approximately one half of 1 percent of generic drug sales. This modest cost should be offset by benefits received by the industry, as faster review times will bring products to market sooner.
Overview of the Proposed Generic Drug User Fee Program
To develop recommendations for a generic drug user fee effective beginning FY 2013, FDA conducted a process that involved the generic drug industry and public stakeholders. In addition to the negotiation sessions with industry trade associations, there were numerous public stakeholder meetings open to all, including industry, patient advocates, consumer advocates, health care professionals, and scientific and academic experts. The final agreement and the goals FDA and industry have agreed to were transmitted to Congress on January 13, 2012.
The Generic Drug User Fee Act (GDUFA) proposal, as negotiated, is aimed at putting FDA’s generic drugs program on a firm financial footing and providing the additional resources necessary to ensure timely access to safe, high-quality, affordable generic drugs. The proposal focuses on quality, access, and transparency. Quality means ensuring that companies, foreign or domestic, that participate in the U.S. generic drug system are held to the same consistent high-quality standards and that their facilities are inspected biennially, using a risk-based approach, with foreign and domestic inspection frequency parity. Access means expediting the availability of low-cost, high-quality generic drugs by bringing greater predictability and timeliness to the review of ANDAs, amendments, and supplements. Transparency means requiring the identification of facilities involved in the manufacture of generic drugs and associated APIs, and improving FDA’s communications and feedback with industry to expedite product access and enhance FDA’s ability to protect Americans in our complex global supply environment.
The additional resources called for under the agreement will provide FDA with the ability to perform critical program functions that could not otherwise occur. With the adoption of user fees and the associated savings in development time, the overall expense of bringing a product to market is expected to decline. The program is expected to provide significant value to small companies and first-time entrants to the generic market. In particular, these companies will benefit significantly from the certainty associated with performance review metrics that offer the potential to dramatically reduce the time needed to commercialize a generic drug, when compared to pre-GDUFA review times.
In addition, the variety of funding sources for the program will ensure that participants in the generic drug industry, whether FDF manufacturers or API [8] manufacturers, whether foreign or domestic, appropriately share the financial expense and benefits of the program. The broad range of funding sources, including and across facility and application types, as well as the large number of each, ensures that individual fees remain reasonable and significantly lower than associated branded drug fees.
As in all of FDA’s other medical product user fee programs, under the proposed generic drug user fee program, user fee funding would supplement appropriated funding to ensure sufficient resources for the Agency’s generic drug review program, and guarantees are in place to ensure that the user fees are supplemental to annual appropriations in the budget.
Biosimilars User Fees
A successful biosimilars review program within FDA will spark the development of a new segment of the biotechnology industry in the United States. The Biologics Price Competition and Innovation Act (BPCI Act) of 2009, which was enacted as part of the Affordable Care Act of 2010, established a new abbreviated approval pathway for biological products shown to be “biosimilar to” or “interchangeable with” an FDA-licensed biological product. With this new abbreviated approval pathway, a biosimilar biologic can be approved by demonstrating, among other things, that it is highly similar to a reference biological product already licensed by FDA. Development of biosimilars is expected to be less risky, less costly, and take less time; therefore, approved biosimilars are expected to be less expensive than the reference product. This program will provide significant benefits for patients, making available more affordable treatments that clinicians will know are biosimilar or interchangeable. The development of this new market segment will expand the opportunities for technical innovation and job growth.
Background
A biosimilar is a biological product that is highly similar to a U.S.-licensed reference product, notwithstanding minor differences in clinically inactive components, and for which there are no clinically meaningful differences between the biosimilar product and the reference product in terms of the safety, purity, and potency of the product.
Under the transition provisions in the BPCI Act, user fees for a biosimilar biological product are assessed under PDUFA. Accordingly, currently, user fees for biological products are the same, regardless of whether the BLA is submitted under the new, abbreviated biosimilar pathway or under the previously existing approval pathway for biological products. However, PDUFA IV expires on September 30, 2012, and the BPCI Act directs FDA to develop recommendations for a biosimilars user fee program for fiscal years 2013 through 2017. To develop these recommendations, FDA consulted with industry and public stakeholders, including patient advocates, consumer advocates, health care professionals, and scientific and academic experts, as directed by Congress. The final recommendations were transmitted to Congress on January 13, 2012.
Program Funding and Metrics
The proposed biosimilars user fee program for FY 2013 to 2017 addresses many of the top priorities identified by public and industry stakeholders and the most important
challenges identified by FDA. The proposed biosimilars user fee program is similar to the PDUFA program in that it includes fees for marketing applications, manufacturing establishments, and products. However, there are some differences because of the nascent state of the biosimilars industry in the United States. For example, there are no
currently marketed biosimilar biological products; accordingly, the recommended biosimilars user fee program includes fees for products in the development phase to generate fee revenue in the near-term and to enable sponsors to have meetings with FDA early in the development of biosimilar biological product candidates.
As in all of FDA’s medical product user fee programs, the proposed biosimilars user fee program supplements appropriated funding to ensure sufficient resources for the Agency’s review programs. Under the proposed biosimilars user fee program, FDA would be authorized to spend biosimilars user fees on Agency activities related to the review of submissions in connection with biosimilar biological product development, biosimilar biological product applications, and supplements. This would include activities related to biosimilar biological product development meetings and investigational new drug applications (INDs). It would also include development of the scientific, regulatory, and policy infrastructure necessary for review of biosimilar biological product applications, such as regulation and policy development, related to the review of biosimilar biological product applications, and development of standards for biological products subject to review and evaluation.
The biosimilars user fee program would support FDA activities at the application stage, such as review of advertising and labeling prior to approval of a biosimilar biological product application or supplement; review of required post-marketing studies and post-marketing studies that have been agreed to by sponsors as a condition of approval; the issuance of action letters that communicate decisions on biosimilar biological product applications; and inspection of biosimilar biological product establishments and other facilities undertaken as part of FDA’s review of pending biosimilar biological product applications and supplements (but not inspections unrelated to the review of biosimilar biological product applications and supplements). Finally, it would support some activities at the post-approval stage, such as post-marketing safety activities, with respect to biologics approved under biosimilar biological product applications or supplements.
CONCLUSION
PDUFA IV expires on September 30, 2012, and FDA is ready to work with you to ensure timely reauthorization of this critical program. If we are to sustain and build on our record of accomplishments, it is critical that the reauthorization occur seamlessly without any gap between the expiration of the old law and the enactment of PDUFA V. The passage of both a new generic drug user fee and a new biosimilars user fee would allow FDA to build upon the success of PDUFA.
Thank you for your contributions to the continued success of PDUFA and to the mission of FDA. I am happy to answer questions you may have.
______________________________________
[1] PDUFA was enacted in 1992 and authorizes FDA to collect fees from companies that produce certain human drug and biological products. Industry agrees to pay fees to help fund a portion of FDA’s drug review activities, while FDA agrees to overall performance goals, such as reviewing a certain percentage of applications within a particular time frame. The current legislative authority for PDUFA expires on September 30, 2012. On January 13, 2012, HHS Secretary Kathleen Sebelius transmitted recommendations to Congress for the next reauthorization of PDUFA.
[2] Milne, Christopher-Paul (2010). PDUFA and the Mission to Both Protect and Promote Public Health [PowerPoint slides]. Presentation at the FDA PDUFA Public Meeting, Rockville, MD.
[3] Scrip NCE Review/Scrip Yearbook/Scrip Magazine (1982 -2005), PharmaProjects R&D Annual Review (2006-2010). New active substances include novel chemical or biological substances not previously approved to treat any disease. There is a close, but not complete overlap, between new active substances and new molecular entities: new active substances exclude radiopharmaceuticals.
[4] “Despite Criticism Of The FDA Review Process, New Cancer Drugs Reach Patients Sooner In The United States Than In Europe,” Samantha A. Roberts, Jeff D. Allen, and Ellen V. Sigal, Health Affairs, June 2011.
[5] “An Economic Analysis of Generic Drug Usage in the U.S.” Independent Analysis by IMS Health, Sept. 2011, http://gphaonline.org/sites/default/files/GPhA%20IMS%20Study%20WEB%20Sep20%2011.pdf.
[6] Drug Master Files are widely used to provide FDA with information about the drug substance, also known as the active pharmaceutical ingredient (API).
[7] An FDF is the final drug product (e.g. tablet, capsule). An FDF is made up of both API(s) and any inactive excipients.
[8} An API is the drug substance responsible for the therapeutic effect (e.g. the chemical aspirin that is combined with excipients to produce the FDF aspirin tablet).