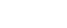About FDA
Initial Results of 510(k) Audit - Analysis of Not Substantially Equivalent (NSE) Determinations
 June 15, 2011
June 15, 2011
Center for Devices and Radiological Health
U.S. Food and Drug Administration
As part of the 510(k) Action Plan published in January 2011, the Center for Devices and Radiological Health (CDRH) committed to post initial results of an audit of the 510(k) program to the FDA website. This initial analysis focuses only on 510(k) submissions that CDRH determined were Not Substantially Equivalent (NSE).
NSE determinations are a subset of 510(k) decisions. For manufacturers, an NSE determination represents an inefficient use of time and resources. For FDA, NSE determinations require significant agency resources and time, yet fail to result in the marketing of a new product.
CDRH performed this analysis to better understand the reasons that 510(k) submissions result in NSE determinations. Between 2001 and 2010, approximately 3.5 percent of 510(k) submissions resulted in an NSE determination. For FY 2010, 8 percent of 510(k) submissions resulted in an NSE determination; the NSE rate has decreased to 5 percent through the first 8 months of FY 2011.
Percent of 510(k) Submissions with an NSE Decision per Year
Reasons for NSE Determinations
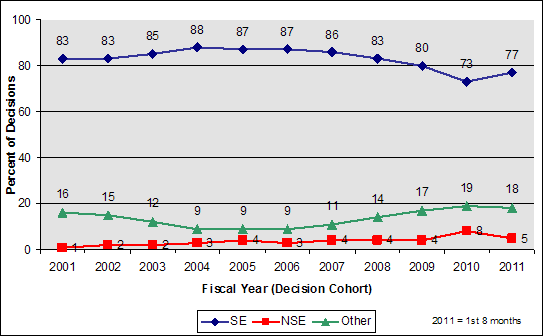
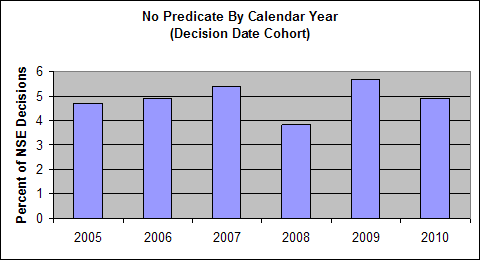
CDRH analysis of NSE decisions from 2005 to 2010 showed four main reasons why 510(k) submissions result in NSE determinations:
- No Predicate - a suitable predicate does not exist;
- New Intended Use - the intended use of the new device is different than the intended use of the predicate;
- New Technology - the technology is not substantially equivalent to the existing technology for the predicate; and
- Lack of Performance Data - there were no performance data provided in the 510(k) submission, the data provided were inadequate, or the data failed to demonstrate that device performance was at least equivalent to the identified predicate.
The following charts show the annual percentage of NSE decisions in each of these four categories. The data shown is based on analysis of more than 700 representative NSE determinations made during calendar years 2005-2010.
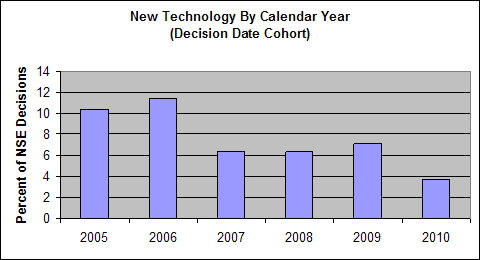
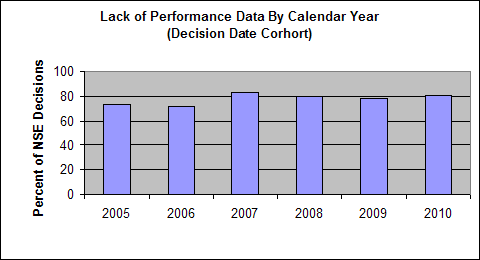
Lack of Performance Data
Data in this analysis show that approximately 80 percent of NSE determinations are due to insufficient performance data because:
- the sponsor failed to provide data or the data provided were inadequate;
- additional data were required due to a new indication, new technology, or a new safety concern; and/or
- the demonstrated performance of the device was inferior to the predicate.
Because a lack of performance data was the most common reason for an NSE determination, additional detailed analysis of 109 510(k) submissions received in FY 2009 and found NSE was performed to determine the factors and circumstances that contributed to these NSE decisions.
Significant findings of this subsequent analysis are:
- In the majority of NSE cases, FDA made repeated requests for data because 510(k) sponsors failed to provide the requested performance data. Specifically, for 88 percent of submissions found to be NSE, additional review cycles were necessary either because the sponsor failed to fully address FDA's original questions or because the sponsor's response raised new safety concerns that they failed to resolve.
- In 18 percent of the submissions, the FDA asked for performance data that had not been required for SE determination of the predicate device. For example, the FDA may have requested additional biocompatibility testing when animal deaths occurred in a study’s test group, but not in the control group. The FDA may have requested additional preclinical testing when adverse event reports raised a new, specific safety issue associated with failure of a material used in the device. The FDA may also have requested clinical data upon identification of a safety concern in the pre-clinical studies.
- In 16 percent of submissions analyzed, the sponsor provided performance data that showed poorer performance than the predicate device.
Overall NSE Analysis
The FDA requested submission of new information that had not previously been requested for a predicate device in 20 percent of NSE determinations. In these cases, the agency requested new information because the device had different indications, a new technology, or a new safety issue that required further evaluation. In all other cases (80 percent) the agency based its NSE determination on the sponsor’s failure to provide adequate data to support an SE determination or the information provided failed to demonstrate at least equivalent device performance compared to that of the predicate.
Review Cycles
This analysis also evaluated the number of review cycles it took reach an NSE determination. As the chart below shows, the FDA has increasingly provided sponsors with numerous attempts to reach SE decisions by engaging in repeated review cycles.
NSE decisions by Cycle
| Final Decision | Cycles 1-3 % NSE | Cycles 4+ % NSE | Max Cycles |
|---|---|---|---|
| 2005 | 93 | 7 | 6 |
| 2006 | 97 | 3 | 4 |
| 2007 | 93 | 7 | 4 |
| 2008 | 89 | 11 | 5 |
| 2009 | 85 | 15 | 7 |
| 2010 | 87 | 13 | 6 |
Conclusions
Although NSE determinations represent a small percentage of all 510(k) decisions, they utilize a greater amount of FDA time and resources than the average 510(k). The absence of a predicate, the introduction of a new intended use, or new technologies account for a minority of NSE determinations. The vast majority of NSE decisions are due to the absence of adequate performance data, sometimes despite repeated FDA requests.
The FDA continues to work interactively with sponsors in an effort to reach an SE determination, which is reflected in a marked increase in the number of submissions reaching NSE determinations after four or more review cycles. Because these efforts divert FDA resources from other work, CDRH will continue to monitor the performance of the 510(k) program, the NSE rate, and the reasons for NSE determinations.