Medical Devices
Proposed Pilot Triage Program
- Program Objectives
- Scope
- General Concepts
- Quick Review Criteria
- Quick Review Decision Summary
- Review and Clearance Process
- Quick Review- Review Time
- Triage Program Metrics
- Pilot Triage Program Training and Quality Assurance
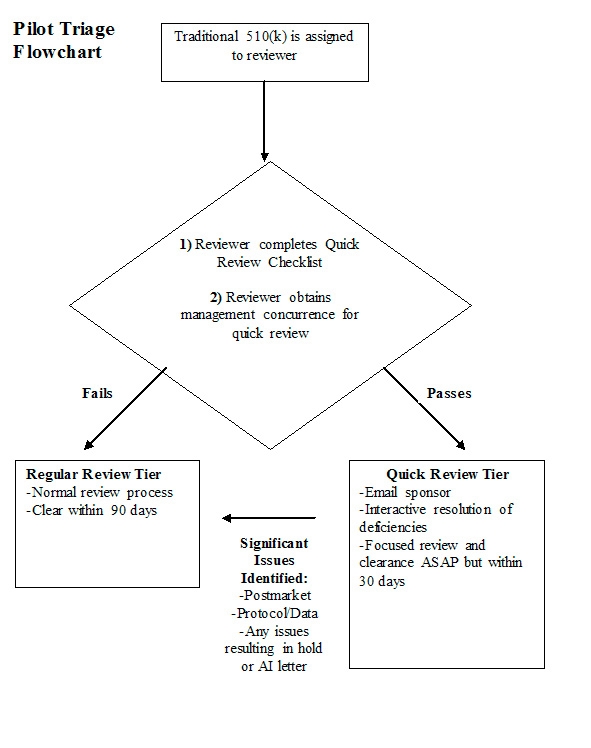
- Pilot Triage Flowchart
- Appendix A
- Appendix B
The Pilot Triage Program is an internal workload management tool. The main objective of this program is to reduce the review time of Traditional 510(k) applications that are of good quality in order to create an incentive to sponsors to submit good quality applications to OIVD. Another objective is to diminish the effort and time reviewers dedicate to Traditional 510(k) applications for products that are well known to the Office. The expected end results are to attain faster product availability and to increase reviewer time for other work-related activities such as training/continuing education, or other regulatory activities.
The Triage Pilot Program will apply to Traditional 510(k) applications that qualify for Quick Review (see Quick Review Criteria below).
Pilot Duration: April 2, 2012- October 2, 2012
- The Program will have 2 tiers: Quick Review and Regular Review tiers:
1. Quick Review Tier – good quality submissions that fit quick review criteria (see quick review criteria below); clear as soon as possible but within 30 days
2. Regular Review Tier - current normal 510(k) review process; clear within 90 days
- The Pilot Triage Program will be applicable to all Traditional 510(k) applications that pass Quick Review criteria.
- Quality of the 510(k) review is maintained in the Triage program, since all elements of the submission will still be reviewed. The faster review time in the Quick Review Tier is achieved by:
- Good quality submissions allowing a shorter review time;
- The reviewers focusing on the key aspects of the submission because of prior experience and knowledge of the device/device performance; and
- Less review documentation produced by the reviewer compared to regular review (see Quick Review Decision Memo) because the sponsor has provided a comprehensive 510(k) Summary.
Quick Review Criteria
The Quick Review criteria emphasize good quality 510(k) applications and FDA experience with the device. Submissions in the Quick Review tier must:
- Pass Quick Review Checklist – This checklist includes elements that address both submission quality and submission content. These elements will be carefully assessed to ensure the submission is well organized, written with clarity, contains all the elements expected to be in the submission, contains the expected studies to support claims, etc. A reviewer may recommend a Regular Review for a submission when all necessary administrative and 510(k) elements are present, but the elements are not of sufficient quality to permit a Quick Review in the targeted time frame (e.g., numerous deficiencies in the clarity, content quality, or other quality aspects of the submission are identified that will most likely need to be resolved through a deficiency letter).
Note: The Quick Review Checklist is not a Refuse-to-Accept Checklist. Once the new Refuse-to-Accept Policy and checklist are finalized as outlined in the MDUFA III commitments, the Quick Review Checklist will be re-assessed to avoid duplicative efforts. The Quick Review Checklist will not be used to refuse-to-accept a submission during the Triage Pilot.
- Pass a Total Product Life Cycle (Postmarket) Search – The reviewer will use internal CDRH databases to identify new or unknown postmarket issues or identify older postmarket issues that have not been addressed appropriately that may influence premarket review. Postmarket issues include recalls, MDRs, and other patient safety/product quality issues.
Any identified postmarket information should be briefed to management for a decision of whether the submission should continue to be considered for Quick Review. If significant postmarket issues are identified, the device is removed from Quick Review and placed in the Regular Review tier.
- Seek clearance for a device for which FDA has review experience and knowledge of expected performance– Devices for which the review division has considerable review experience and also knowledge of expected device performance are ideal for Quick Review. FDA experience and knowledge is a broad term that can encompass any of the following: the review division has reviewed at least a few similar 510(k) applications, the expected performance is known, the intended use/technology/analyte is well established, the division knows the type of data/information that needs to be included in the submission, there is a FDA guidance document and/or applicable well accepted CLSI standard available and the sponsor has adhered to the guidance document/standard, etc.
- Not need an extensive consult to complete 510(k) review – Extensive consults may include statistical, clinical, etc. Addition of extensive consults to the review process will add to review time, making Quick Review and goal of clearance within 30 days very difficult.
Note: Informal consults (e.g. quick questions via e-mail or in person) with product experts/specialists, team leaders, etc., and non-extensive consults that can be completed in a short period of time are acceptable for Quick Review.
- Contain a 510(k) Summary [and not a 510(k) Statement] – Sponsors may either submit a 510(k) Summary or 510(k) Statement with their Traditional 510(k) submissions. Currently, upon clearance of a submission, OIVD posts the 510(k) Statement or 510(k) Summary online along with an OIVD Decision Summary document. Unlike a 510(k) Statement, the 510(k) Summary contains a summary of the information upon which the manufacturer based their SE claim, which is also provided in the OIVD Decision Summary – including regulatory information, intended use, device description, device name, predicate information, applicant information, discussion of non-clinical and clinical tests, and a substantial equivalence discussion. The intent of the Quick Review process is to take advantage of the information already provided by the sponsor in the 510(k) Summary and write a non-public Decision Memo that does not repeat this information and therefore takes less time to write. For this reason, only submissions containing 510(k) Summaries will qualify for Quick Review; a new Quick Review Decision Summary document will be drafted to serve as the internal Review Memo for the Quick Review process.
The Quick Review Decision Memo will serve as the internal Review Memo for Quick Review qualifying submissions. This approach will reduce review time because the good quality assessment will be completed early in the Quick Review process through the Quick Review Checklist contained within the Quick Review Decision Memo. Additionally, the 510(k) Summary provided by the sponsor already provides basic submission, regulatory and substantial equivalence information; therefore, reviewers will not have to re-write this information in a review memo. This decrease in review documentation will help decrease the review time of the submission, will diminish reviewer effort, and will promote faster availability of the product without compromising the quality of the review. The Quick Review Decision Memo will not be posted online for the public. Instead, a Quick Review boilerplate Statement (see Appendix B) will be posted online with the 510(k) Summary.
1. The 510(k) application comes to the review division/branch and the submission is assigned to the reviewer. The reviewer makes a preliminary determination of whether the submission is a potential candidate for Quick Review using the Quick Review Checklist. This first pass of Quick Review assessment should take place within 1 week of the submission being assigned.
2. If the reviewer identifies the submission as a candidate for Quick Review, the reviewer will contact the product specialist/expert and management to obtain concurrence that the submission qualifies for quick review. Concurrence can be obtained by e-mail or by setting up a brief meeting with the product specialist/management if necessary.
3. If there is concurrence that the submission should undergo Quick Review, the reviewer conducts a focused review of the submission (including product labeling) and works interactively with the sponsor to clarify any minor issues. There should be no need for substantial additional information during Quick Review. If significant issues are identified and/or the submission needs to be placed on hold, the submission should be removed from Quick Review and placed in the Regular Review tier.
At the start of focused review, the reviewer will let the sponsor know (e.g., via e-mail) that because their submission is of good quality, etc., the submission has qualified for Quick Review. The Quick Review process will be explained and it will be stated that he or she would like to work interactively with the sponsor to resolve any minor issues to expedite clearance. The sponsor will also be notified in this communication that if any major issues are identified, the submission may be placed on hold and it may no longer qualify for Quick Review. Refer to Appendix A for sample e-mails for submissions that qualify for Quick Review
4. Quick Review submissions should be cleared as soon as possible but within 30 days. If at Day 30 the submission is still not close to being cleared (e.g., late issues were identified and a hold letter needs to be sent, sponsor doesn’t respond quickly to real-time interactive questions, etc.), management should be notified and it should be determined whether the submission still qualifies for Quick Review.
5. The reviewer uses the Quick Review Decision Memo as internal review documentation to support clearance.
6. The boilerplate Quick Review Statement is posted online with the 510(k) Summary after clearance is granted.
- Reviewer does first pass review of submission and Quick Review Criteria: 1 week
- Management concurrence of quick review tier determination: 1-3 days after being contacted by the reviewer
- Reviewer conducts focused review of the submission to clear the submission ASAP but within 30 days. If not cleared within 30 days – the reviewer, along with management should rethink whether the submission still belongs in the Quick Review tier.
OIVD will collect the following information to gauge the success of the program:
- Total FDA review time for Quick Review vs. Regular review
- Number of submissions accepted into Quick Review tier
- Number of submissions that are cleared via Quick Review
- Number of submissions converted from Quick Review to Regular review
- Reasons why submission are not accepted into the Quick Review or are converted to a Regular review
Pilot Triage Program Training and Quality Assurance:
- All reviewers and managers will be trained on the key elements of the program
- A Quality Assurance group will be formed consisting of representation from each review division to monitor the implementation of the Pilot Triage Program, obtain feedback from reviewers/managers, answer questions about the program, and make program adjustments as needed.
Pilot Triage Flowchart
The following are a set of e-mail templates that reviewers can use during the Triage review process. The e-mails can be customized as appropriate. They are intended to help explain the purpose of the Triage program and give reviewers a starting point for communicating with sponsors.
1. E-mail for Acceptance into Quick Review Tier
Subject: [kxxxxxx] – [Sponsor] [Trade Name]
Dear [sponsor],
The Center for Devices and Radiological Health (CDRH) of the Food and Drug Administration (FDA) has received the 510(k) referenced above. It appears that the content and quality of the submission may be sufficient for this submission to be considered in the OIVD Pilot Triage-Quick Review program. Please note that acceptance into the Triage-Quick Review program is contingent upon your submission of a comprehensive 510(k) Summary (e.g., similar in detail to web-posted OIVD Decision Summaries).
The objective of this program is to reduce review time of good quality 510(k) applications that meet all Triage-Quick Review criteria and to facilitate a quicker and more focused review by the Agency.
Our goal is to work interactively with you to complete the review ASAP but within a total of 30 days. We will let you know of any requests for clarification or additional information. Please note that if major deficiencies are identified as the submission undergoes further review, this submission may no longer be considered for Triage-Quick Review. You will be notified promptly if this situation occurs.
If you have any questions regarding this pilot Triage-Quick Review program, please do not hesitate to contact me.
Thank you,
[Reviewer Name]
2. Email for Minor Deficiencies
Subject: [kxxxxxx] – [Sponsor] [Trade Name]
Dear [sponsor],
The Center for Devices and Radiological Health (CDRH) of the Food and Drug Administration (FDA) has completed an initial review of the 510(k) referenced above under the Triage-Quick Review Program. To complete the review of your submission, we are requesting the following additional information:
1. …..
2. …..
We believe that these issues can be resolved interactively in a timely manner. Your submission is not currently being placed on hold. You can send me your response by e-mail.
Please note that if additional deficiencies are identified as the submission undergoes further review or if we are unable to resolve these deficiencies in a timely manner, this submission may be placed on hold or no longer be considered for Triage-Quick Review.
If you have any questions regarding this request for additional information or the pilot Triage-Quick Review program, please do not hesitate to contact me.
Thank you,
[Reviewer Name]
- E-mail for Conversion from Quick Review to Regular Review
Subject: [kxxxxxx] – [Sponsor] [Trade Name]
Dear [sponsor],
This e-mail is to notify you that we have identified the following significant deficiency(ies) in the 510(k) submission referenced above.
1. ………
2. ………
Therefore, your submission is being taken out of OIVD’s Pilot Triage Program and is being converted from Quick Review to Regular Review. Please note that we are not placing your 510(k) on hold at his time. We will continue our review of your submission as we would normally and communicate any additional deficiencies to you interactively and/or once our review is complete.
If you have any questions regarding this e-mail, please do not hesitate to contact me.
Thank you,
[Reviewer Name]
Statement for the Record, K________
This 510(k) was reviewed under OIVD’s Pilot Triage Program. This program represents an internal workload management tool intended to reduce internal FDA review resources for 510(k) applications that are of good quality upon receipt by FDA.
The information in the 510(k) is complete and supports a substantial equivalence (SE) determination. Please refer to the applicant’s 510(k) summary for a summary of the information that supports this SE determination.