9. Lipids and Lipoproteins
INTRODUCTION
This section of the Guidelines provides
recommendations to pediatric care providers on lipid management in their
patients. The section begins with background information about the association
between dyslipidemia and atherosclerosis and the changing clinical picture of
dyslipidemia in childhood. This is followed by the Expert Panel's written
synopses of the evidence review relative to lipids in five subsections:
- Relationship between dyslipidemia and
atherosclerosis
- Lipid and lipoprotein assessment in childhood and
adolescence
- Overview of the dyslipidemias
- Dietary treatment of dyslipidemias
- Pharmacologic treatment of dyslipidemias
This evidence review and the development process for
the Guidelines are outlined in Section I. Introduction and are described in
detail in Appendix A. Methodology. As described, the evidence review here
augments a standard systematic review where the findings from the studies
reviewed constitute the only basis for recommendations with each study
described in detail. This evidence review combines a systematic review with an
Expert Panel consensus process that incorporates and grades the quality of all
relevant data based on preidentified criteria. Because of the large volume
constituted by the included studies and the diverse nature of the evidence, the
Expert Panel also provides a critical overview of the studies reviewed for each
of the five subsections, highlighting those that in its judgment provide the
most important information. Detailed information from each study has been
extracted into the evidence tables, which will be available at
http://www.nhlbi.nih.gov/guidelines/cvd_ped/index.htm.
The conclusions of the Expert Panel's review of the evidence are summarized and
graded at the end of each subsection, followed by age-specific recommendations.
Where evidence is inadequate, recommendations are a consensus of the Expert
Panel. References are listed sequentially at the end of this section, with
references from the evidence review identified by unique PubMed identifier
(PMID) numbers in bold text. Additional references do not include the PMID
number.
BACKGROUND
Since the previous guidelines for lipid management in
children and adolescents from the National Cholesterol Education Program (NCEP)
were published in 1992,[1] both the knowledge base surrounding
dyslipidemia in childhood and the clinical picture have changed. A series of
critical observational studies, which are summarized below, have demonstrated a
clear correlation between lipoprotein disorders and the onset and severity of
atherosclerosis in children, adolescents, and young adults.[2],[3],[4]
Over that time period, a major increase in the prevalence of obesity has led to
a much larger population of children with dyslipidemia. At the time of the
original guidelines, the focus was almost exclusively on identification of
children with elevated low-density lipoprotein cholesterol (LDL–C). Since
then, the predominant dyslipidemic pattern in childhood is a combined pattern
associated with obesity, with moderate to severe elevation in triglycerides
(TG), normal to mild elevation in LDL–C, and reduced high-density
lipoprotein cholesterol (HDL–C). Both dyslipidemic patterns have been
shown to be associated with initiation and progression of atherosclerotic
lesions in children and adolescents, as demonstrated by pathology and imaging
studies.[2],[3],[4],[5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15]
Identification of children with dyslipidemias, which place them at
increased risk for accelerated early atherosclerosis, must include a
comprehensive assessment of serum lipids and lipoproteins.
OVERVIEW OF THE EVIDENCE FOR A RELATIONSHIP BETWEEN
DYSLIPIDEMIA AND ATHEROSCLEROSIS
Postmortem pathology studies of atherosclerosis in
children, adolescents, and young adults demonstrate that early atherosclerotic
lesions of fatty streaks and fibrous plaques are significantly related to
elevations in total cholesterol (TC), LDL–C, and non-HDL–C; lower
levels of HDL–C; and the presence and intensity of other risk factors.[2],[3],[4],[5],[6],[7]
The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study,
described in detail in Section II. State of the Science, evaluated the presence
of atherosclerosis at postmortem in adolescents and young adults ages
15–34 years who died accidentally.[3],[4],[5]
The extent of atherosclerosis in the aorta and coronary arteries was correlated
with the presence of abnormal lipid levels, obesity, a measure of hypertension,
and evidence of cigarette smoking. Using a risk score derived from these
results, non-HDL–C was shown to be the major correlate of coronary
atherosclerosis in this age group, with a 30 milligram per deciliter (mg/dL)
increase in non-HDL–C equivalent to 2 years of vascular aging.[6],[7]
Based on imaging studies assessing subclinical atherosclerosis, abnormal levels
of lipids and lipoproteins are associated with endothelial dysfunction assessed
by flow-mediated dilation (FMD) in the brachial artery, coronary artery calcium
(CAC), and increased carotid intima-media thickness (cIMT)—all of which
are considered precursors of advanced atherosclerosis.[8],[9],[10],[11],[12],[13],[14],[15]
Childhood levels of TC/HDL–C, LDL–C, HDL–C, and TG are each
predictors of CAC and cIMT.[9],[11],[12]
Overall risk factor scores, lipids, and increased body mass index (BMI)
have been shown to be significant longitudinal predictors of CAC and increased
cIMT.[16] In subjects from the
Cardiovascular Risk in Young Finns (Young Finns) Study, the baseline
cardiovascular (CV) risk profile predicted both CAC and cIMT from adolescence
through young adulthood. The Young Finns study also found that dyslipidemia in
childhood with elevated LDL–C and TG levels predicted increased cIMT
independently and synergistically with other CV disease (CVD) risk factors and
the metabolic syndrome. Young adults with a TC level >200 mg/dL had five
times the risk of developing CVD events 40 years later compared with those who
had a TC level <172 mg/dL.[16] As part of the metabolic
syndrome, childhood dyslipidemia has been shown to predict development of the
metabolic syndrome, type 2 diabetes and adult CV disease at 25 year
follow-up.[17],[18]
The effects of risk factors, including dyslipidemia, on coronary
lesion severity are multiplicative rather than simply additive.[2]
In adults with elevated LDL–C but without CVD,
convincing evidence suggests that lipid-lowering therapy with statins
significantly decreases the incidence of major coronary and cerebrovascular
events.[19] In children, no randomized
clinical trials (RCTs) address whether treating dyslipidemias in children and
adolescents will reduce CVD events in later life. However, increasing evidence
indicates that lipid-lowering interventions in childhood delay the
atherosclerotic process. In one trial, healthy male children treated with a low
saturated fat, low cholesterol diet from infancy had enhanced vascular
endothelial function at age 11 years compared with controls; this effect was
not seen in females.[20] In a separate 2-year study,
lowering TC and LDL–C levels with a low-fat diet and statin therapy in
children and adolescents with heterozygous familial hypercholesterolemia (FH)
was associated with a significantly smaller increase in cIMT than that seen in
children treated with diet and placebo.[21]
Followup of participants in this trial who continued on statin therapy for a
mean of 4.5 years revealed that younger age at initiation was associated with
subsequently smaller cIMT, suggesting that earlier initiation of statin therapy
delays progression of the atherosclerotic process in children with FH assessed
noninvasively.[22] In another RCT, impaired
endothelial function in FH children, as judged by FMD, improved significantly
in those with LDL–C lowered by simvastatin therapy versus those on placebo
to a level similar to that in normal non-FH controls.[23]
OVERVIEW OF THE EVIDENCE FOR LIPID AND LIPOPROTEIN
ASSESSMENT IN CHILDHOOD AND ADOLESCENCE
In the past, NCEP guidelines were based on standard
serum measures of TC, very low-density lipoprotein cholesterol (VLDL–C),
HDL–C, LDL–C, and TG, with recommendations focused on TC and
LDL–C. Since that time, knowledge about lipoprotein heterogeneity and
apolipoproteins as predictors of CVD has increased significantly. This evidence
review assessed whether measures of any of these in youths are better
predictors of subclinical atherosclerosis in adults.
Apolipoproteins B and A–1
In adults, apolipoprotein B (apoB), the major
apolipoprotein of LDL–C, and apolipoprotein A–I (apoA–1), the
major apolipoprotein of HDL–C, are predictors of the development of CVD
and response to treatment to prevent CVD.[24] The level of
total apoB includes all the apoB-containing lipoproteins, chylomicrons, and
VLDL–C and their remnants: intermediate-density lipoprotein
cholesterol (IDL–C), LDL–C, and lipoprotein(a) (Lp(a)). When present
in increased amounts, all the apoB-containing lipoproteins are considered
atherogenic. Since there is one molecule of apoB on each apoB-containing
lipoprotein particle, apoB provides the most accurate assessment of the total
number of LDL–C particles. ApoB and apoA–1 are determined using
well-standardized immunochemical methods.[24],[25] These apolipoproteins have been
studied in children and adolescents; cut points for apoB and
apoA–1—empirically derived from the Third National Health and
Nutrition Examination Survey (1988–1994) (NHANES III)—are shown in
Table 9–1.[25]
In the Bogalusa Heart Study, tracking of apoB and
apoA–1 over 4 years was compared with tracking for LDL–C and
HDL–C. The correlations for apoB and apoA–1 were significant but of
somewhat lower magnitude than those for LDL–C and HDL–C.[26] Thus, on a population basis,
there was no clear advantage of using apoB and apoA–1 over LDL–C and
HDL–C to assess tracking. Measurement of apoB and apoA–1 and the
ratio of apoB to apoA–1 might provide additional useful information for
selective screening, particularly in youths with a family history of premature
CVD in parents.[27],[28] This may be
related to the fact that elevated apoB is often the first expression of
familial combined hyperlipidemia (FCHL) in adolescents and young adults, before
the onset of overt combined dyslipidemia.[29] In the Bogalusa
study, no improved prediction of cIMT over that obtained with LDL–C and
TC/HDL–C was observed when apoB, apoA–1, or the apoB/apoA–1
ratios were used.[8] However, the latest report from
the Young Finns study indicates that apoB and apoA–1 levels in childhood
were both better predictors of cIMT and brachial endothelial function in adult
life than were LDL–C or HDL–C levels.[14]
Non-HDL–C
Non-HDL–C has emerged as a useful combined
measure of the cholesterol content of all the atherogenic apoB-containing
lipoproteins. TC and HDL–C can be measured accurately in plasma from
nonfasting patients with non-HDL–C calculated by subtracting HDL–C
from TC. The coefficient of variability for non-HDL–C thus reflects the
variability of measuring both TC and HDL–C. This variability is
theoretically less than that for estimated LDL–C, which includes the
variability from the measurement of TC, HDL–C, and TG. Percentiles and
NCEP-equivalent cut points for non-HDL–C have been determined in children
from the Bogalusa study and are shown in Table 9–1.[30]
In adults, non-HDL–C has been shown to be a
better independent predictor of CVD than LDL–C.[31]
In a longitudinal cohort of subjects (N = 1,163) from the Bogalusa study,
studied as both children ages 4–5 years old and adults 27 years later,
non-HDL–C (p = 0.52) and LDL–C (p = 0.58) were the best predictors of
adult levels.[32] The odds ratios (ORs) of developing
dyslipidemia in adulthood, on the basis of childhood levels of non-HDL–C
and LDL–C, were 4.49 and 3.46, respectively, independent of baseline BMI
and BMI change over 27 years. At equivalent cut points, childhood high-risk
non-HDL–C and LDL–C levels were significantly associated with
increased obesity, high LDL–C, and high TG in adulthood. However, only
childhood high-risk non-HDL–C status was associated with low HDL–C,
hyperinsulinemia, and, marginally, hyperglycemia. Thus, childhood
non-HDL–C appears to predict adult dyslipidemia, as well as nonlipid CVD
risk factors, better than LDL–C.[32]
In the pathology studies reported in the PDAY study,
non HDL–C and HDL–C levels were the best lipid predictors of
pathologic atherosclerotic lesions, both significantly associated with fatty
streaks in the thoracic aorta and abdominal aorta and in the right coronary
artery and with raised lesions in all three sites;[33]
non-HDL–C and HDL–C levels were more strongly associated with
pathologic lesions than either apoB or apoA–1.
In the Bogalusa study, levels of non-HDL–C,
LDL–C, TC/HDL–C, apoB, and apoB/apoA–1 in childhood emerged as
significant predictors of subclinical atherosclerosis assessed by higher cIMT
measurements in adulthood, but ORs were highest for LDL–C and
non-HDL–C.[15] Overall, childhood
non-HDL–C was as good as, or better than, other lipoprotein measures in
predicting cIMT in adulthood.
Apolipoprotein E Polymorphism
Apolipoprotein E (apoE) binds to receptors on the
surface of liver cells, promoting the hepatic uptake of remnant lipoproteins of
both dietary and hepatic origins. Human apoE exists as three major
isoforms—E2, E3, and E4—each of
which is specified by an independent allele at the locus for the apoE gene.
Children with the rarest allele, apoE–2, generally have lower levels of TC
and LDL–C, lower BMI and percentage of body fat, and lower insulin but
higher HDL–C levels than those with apoE–3 or apoE–4.[34],[35],[36]
Tracking of plasma lipid and lipoprotein is influenced to some degree by the
apoE polymorphism. Children with apoE–4 have the highest LDL–C
levels, but apoE–4 does not appear to influence the response to a
low-cholesterol, low-fat diet[36] or to the addition of plant
stanols.[37]
Lp(a) Lipoprotein
Lp(a) consists of a molecule of LDL–C in which
its apoB moiety is connected through a disulfide bond to apo(a), a glycoprotein
homologous to plasminogen. When present in elevated amounts, Lp(a) appears to
be atherogenic because of its high cholesterol content and thrombogenic by
virtue of the inhibition of the conversion of plasminogen to plasmin at the
surface of endothelial cells.[38] Lp(a) is most accurately
measured by an enzyme-linked immunosorbent assay (or ELISA) that is independent
of apo(a) size differences, with the upper limit of normal by this method being
75 nanomoles per liter.
In adults, higher Lp(a) levels may be an independent
risk factor for coronary artery disease (CAD), pulmonary vascular disease,
ischemic stroke, and aortic aneurysm.[39] Elevated Lp(a)
levels appear to particularly contribute to risk when combined with high
LDL–C levels. In some families, isolated elevated Lp(a) levels have been
seen with premature CAD and normal lipid and lipoprotein levels. In the
Bogalusa study, Lp(a) was measured in 2,438 children.[40] Mean Lp(a) levels were 1.7
times higher in Blacks than in Whites. White children with a history of
parental myocardial infarction had significantly higher Lp(a) levels than did
those with a negative family history, but there was no such association in
Black children. Nowak-Gottl studied 1,002 household members of 282 White
pediatric patients with a first acute ischemic stroke. Significant heritability
estimates (but not environmental estimates) were found for Lp(a).[41] In children with stroke, Lp(a)
levels are significantly elevated in about half of cases with either ischemic
or hemorrhagic stroke.[42]
Advanced Lipoprotein Testing
The plasma levels of VLDL–C, LDL–C, and
HDL–C subclasses and their sizes have been determined in children and
adolescents by nuclear magnetic resonance spectroscopy[43],[44],[45] and by vertical-spin
density-gradient ultracentrifugation[46] in research studies,
but cut points derived from these methods for the diagnosis and treatment of
dyslipidemia in youths are not currently available.
OVERVIEW OF THE EVIDENCE FOR NORMAL DISTRIBUTION
PATTERNS OF LIPIDS AND LIPOPROTEINS
The Lipid Research Clinics (LRC) Prevalence Study
collected lipid and lipoprotein values in children and adolescents from ages 0
to 19 years at multiple centers in the United States and Canada from 1970 to
1976. In that study, the mean TC level was approximately 160 mg/dL, and the
mean LDL–C level was 100 mg/dL. The 95th percentiles for these two
measures were 200 mg/dL for TC and 130 mg/dL for LDL–C.[47]
These values were used in developing recommendations in National
Cholesterol Education Program: Report of the Expert Panel on Blood
Cholesterol Levels in Children and Adolescents, which was published in
1992.[1]
The NHANES III collected cholesterol levels in more
than 7,000 U.S. children ages 0–19 years from 1988 to 1994.[48] Over the intervening time
period following the LRC study—just over a decade—lipid levels in the
pediatric population had increased significantly. The mean TC level was 171
mg/dL, and the 95th percentile was 216 mg/dL; the 95th percentile for
LDL–C was 152 mg/dL. This evaluation included significant numbers of
African American, Hispanic American, and Mexican American subjects. African
American children and adolescents were shown to have significantly higher TC
and HDL–C levels and lower TG levels compared with the other racial/ethnic
groups of children in the survey.[48] Although the
percentiles of lipid levels varied by race, the risk of atherosclerosis (as
measured by cIMT) was equally related to lipid levels and risk factors in
African Americans and Whites, so results were not reported separately.[10]
Lipid levels change with normal growth and maturation.
Lipoproteins are very low in cord blood at birth and rise slowly in the first 2
years of life.[49],[50] After age 2
years, lipid and lipoprotein levels are relatively stable until adolescence.
During puberty, TC and LDL–C levels decrease with increasing age before
rising in the late-teen years and again in the third decade of life.[51] HDL–C levels decrease
during puberty in males but not in females. From the Bogalusa study, there are
differences in lipoprotein levels between Blacks and Whites during childhood,
with higher levels of TC and HDL–C and lower levels of VLDL–C and TG
in Black children and adolescents.[52] Recent
evaluations have developed age- and gender-specific distribution curves for
lipoproteins from the NHANES III data linked to CVD risk.[53],[54]
The distribution curves reflect the changes noted with normal growth and
maturation. It has been suggested that these lipid curves, similar to growth
curves, be used to account for normal maturational changes and to allow
accurate selection of high-risk thresholds. Alternatively, designating the 50th
percentile of the pooled NHANES results as "borderline high" and the 75th
percentile as "high," results in thresholds similar to these derived values.
The cut points for plasma lipid, lipoprotein, and apolipoprotein levels in
children and adolescents are shown in Table 9–1 and for young adults in
Table 9–2.
Table 9–1. Acceptable, Borderline-High, and High
Plasma Lipid, Lipoprotein and Apolipoprotein Concentrations (mg/dL) For
Children and Adolescents*
NOTE: Values given are in mg/dL. To
convert to SI units, divide the results for total cholesterol (TC), low-density
lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C),
and non-HDL-C by 38.6; for triglycerides (TG), divide by 88.6.
|
Category |
Acceptable |
Borderline |
High+ |
|
TC |
< 170 |
170-199 |
≥ 200 |
|
LDL-C |
< 110 |
110-129 |
≥ 130 |
|
Non-HDL-C |
< 120 |
120-144 |
≥ 145 |
|
ApoB |
<
90
|
90-109
|
≥ 110
|
|
TG |
|
|
|
|
0-9 years |
< 75 |
75-99 |
≥ 100 |
|
10-19 years |
< 90 |
90-129 |
≥ 130 |
|
Category |
Acceptable |
Borderline |
Low+ |
|
HDL-C |
> 45 |
40-45 |
< 40 |
|
ApoA-I |
>120 |
115-120 |
<115 |
* Values for
plasma lipid and lipoprotein levels are from the National Cholesterol Education
Program (NCEP) Expert Panel on Cholesterol Levels in Children.1 Non-HDL-C
values from the Bogalusa Heart Study are equivalent to the NCEP Pediatric Panel
cut points for LDL-C.[30] Values for plasma apoB and apoA-1
are from the National Health and Nutrition Examination Survey III.
+ The cut points for
high and borderline high represent approximately the 95th and 75th percentiles,
respectively.[1],[25],[30]
Low cut points for HDL-C and apoA-1 represent approximately the 10th
percentile.[25]
Table 9-2. Recommended Cut Points for Lipid and
Lipoprotein Levels (mg/dL) in Young Adults*
NOTE: Values given are in mg/dL. To convert to SI
units, divide the results for total cholesterol (TC), low-density lipoprotein
cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and
non-HDL-C by 38.6; for triglycerides (TG), divide by 88.6.
|
Category |
Acceptable |
Borderline High |
High |
|
TC |
<190 |
190-224 |
≥225 |
|
LDL-C |
<120 |
120-159 |
≥160 |
|
Non-HDL-C |
<150 |
150 -189 |
≥190 |
|
TG |
<115 |
115-149 |
≥150 |
|
Category |
Acceptable |
Borderline Low |
Low |
|
HDL-C |
>45 |
40-44 |
< 40 |
* Values
provided are from the Lipid Research Clinics Prevalence Study.[47] The
cut points for TC, LDL-C, and non-HDL-C represent the 95th percentile for
subjects ages 20-24 years and are not identical with the cut points used in the
most recent National Cholesterol Education Program's Adult Treatment Panel
III,[55] which are derived from combined data
on adults of all ages. The age-specific cut points given here are provided for
pediatric care providers to use in managing this young adult age group. For TC,
LDL-C, and non-HDL-C, borderline high values are between the 75th and 94th
percentiles, whereas acceptable values are <75th percentile. The high TG cut
point represents approximately the 90th percentile, with borderline high
between the 75th and 89th percentiles; acceptable is <75th percentile. The
low HDL-C cut point represents roughly the 25th percentile, with borderline low
between the 26th and 50th percentiles; acceptable is >50th percentile.
OVERVIEW OF THE EVIDENCE FOR TRACKING OF LIPID AND
LIPOPROTEIN LEVELS FROM CHILDHOOD INTO ADULT LIFE
An important factor in considering lipid assessment in
childhood is the accuracy of childhood lipid levels in predicting adult
results. This evidence review identified 13 prospective screening cohort
studies that assessed tracking of elevated lipid and lipoprotien levels from
childhood into adulthood, with significant tracking identified in 12 of the 13
studies. From the Bogalusa study, more than 3,000 children ages 5–14 years
at baseline were followed for 12 years. Lipid and lipoprotein levels tracked
well statistically, with the best correlations for TC and LDL–C levels
after age 12 years. Children with TC levels above the 75th percentile had
approximately a 50 percent rate of falling into a similar percentile as adults,
which was more than twice that predicted by chance alone.[51],[56]
The best predictors of elevated TC and LDL–C levels in adults were
childhood elevations in TC and LDL–C levels.[56]
In a stepwise multiple logistic regression, incremental increases in TC and BMI
independently predicted incremental increases in adult TC.[53]Similarly, in a later report from the
Bogalusa study, obesity, insulin level, and TC level were highly correlated,
and increasing levels of obesity predicted elevated lipid levels.[57]
The 16-year experience of the Beaver County Lipid
Study also demonstrated that the overall correlation (r = 0.44) between
baseline and followup TC levels was significant; females had a higher
correlation than males (0.51).[58] In an RCT of dietary
intervention in children ages 7 months through 5 years, tracking of TC
levels was significant for both the diet group and the control group, and the
only gender effect was stronger HDL–C tracking for boys.[34]
In an epidemiologic study of Finnish children followed for more than 12 years,
all lipid and lipoprotein levels had significant tracking, with correlations
ranging from 0.48 to 0.59 for TC, LDL–C, and HDL–C.[59]
In the LRC study,[47] more than 1,700
subjects had their initial TC levels drawn in grades 1–12 and then again
30 years later. Sensitivities for elevated TC and LDL–C levels were 44
percent and 43 percent, respectively, and specificities were 85 percent and 86
percent, respectively. Sensitivity and specificity were not improved by
selecting children with a positive family history of early CVD or high
cholesterol level.[60] Pubertal changes caused
sensitivities and specificities to be lowest at ages 14–16 years
regardless of lipid status. CVD events were too infrequent to allow testing of
the ability of childhood cholesterol levels to predict future CVD in a still
relatively young adult cohort.
The Muscatine Study followed more than 14,000 children
with two measures of TC levels and other risk factors, the first between ages 8
and 18 years and the followup between 20 and 30 years later. Although TC
tracked well from adolescence to adulthood, many adolescents identified as high
risk would not be considered high risk in adulthood.[61]
For children with TC levels above the 75th percentile on two occasions, 75
percent of females and 56 percent of males would not qualify for treatment as
adults. For children with TC levels above the 90th percentile on two occasions,
43 percent of females and 70 percent of males had lipid levels above the 75th
percentile as adults—that is, the level designated as requiring
intervention in adults.[61]
In summary, the vast majority of epidemiologic studies
indicate that there is strong statistical tracking of TC and LDL–C levels
from childhood to adulthood. Clinically, this means that approximately half of
children with lipid levels above the 75th percentile in childhood will have
elevated lipid levels as adults.[62] In general, the
higher the childhood result and the older the postpubertal age at which the
value is obtained, the better the correlation with results in adult life:
A TC level above 200 mg/dL will identify children at risk for more marked
hypercholesterolemia with 90 percent confidence.[63]
OVERVIEW OF THE EVIDENCE FOR DYSLIPIDEMIAS IN
CHILDHOOD AND ADOLESCENCE
Dyslipidemias are abnormalities in lipoprotein
metabolism associated with any abnormal level of lipoproteins. There are many
different types of dyslipidemias, which are influenced by genetics and
environmental factors, including nutrition, physical inactivity, smoking,
social factors, etc. Dyslipidemia also can be secondary to other specific
causes that affect lipoprotein metabolism; these are listed in Table 9–3.
The presence of dyslipidemia is an established risk factor for the development
of atherosclerosis in both children and adults, but the incidence of CV
clinical events due to atherosclerosis is extremely rare in children.
Table 9–3. Causes of Secondary Dyslipidemia
- EXOGENOUS
- Alcohol
- Drug therapy:
- Corticosteroids
- Isoretinoin
- Beta-blockers
- Some oral contraceptives
- Select chemotherapeutic agents
- Select antiretroviral agents
- ENDOCRINE/METABOLIC
- Hypothyroidism/hypopituitarism
- Diabetes mellitus types 1 and 2
- Pregnancy
- Polycystic ovary syndrome
- Lipodystrophy
- Acute intermittent porphyria
- RENAL
- Chronic renal disease
- Hemolytic uremic syndrome
- Nephrotic syndrome
- INFECTIOUS
- Acute viral/bacterial infection*
- Human immunodeficiency virus infection (HIV)
- Hepatitis
- HEPATIC
- Obstructive liver disease/cholestatic
conditions
- Biliary cirrhosis
- Alagille syndrome
- INFLAMMATORY DISEASE
- Systemic lupus erythematosis
- Juvenile rheumatoid arthritis
- STORAGE DISEASE
- Glycogen storage disease
- Gaucher's disease
- Cystine storage disease
- Juvenile Tay-Sachs disease
- Niemann-Pick disease
- OTHER
- Kawasaki disease
- Anorexia nervosa
- Post solid organ transplantation
- Childhood cancer survivor
- Progeria
- Idiopathic hypercalcemia
- Klinefelter syndrome
- Werner's syndrome
*Delay
measurement until ≥ 3 weeks postinfection.
The known dyslipidemias are defined by age, gender,
and racial cutoffs based on population distributions and known genetic
disorders and are outlined in Table 9–4. Genetic lipid disorders include
FH, FCHL, familial defective apoB (FDB), familial hypertriglyceridemias, and
hypoalphalipoproteinemia. The genetic disorders may be the result of a
single-gene defect but more commonly are due to oligogenic defects involving
several more genes, which lead to abnormal lipoprotein metabolism.[16]
Table 9–4. Summary of Major Lipid Disorders in
Children and Adolescents
|
Primary Lipid Disorders |
Lipid/Lipoprotein Abnormality |
|
Familial hypercholesterolemia |
Homozygous: ↑↑ LDL-C
Heterozygous: ↑ LDL-C* |
|
Familial defective apolipoprotein B |
↑ LDL-C |
|
Familial combined hyperlipidemia* |
Type IIa: ↑ LDL-C
Type IV: ↑
VLDL-C, ↑ TG
Type IIb: ↑ LDL-C, ↑ VLDL-C, ↑ TG
Types IIb and IV often with ↓ HDL-C |
|
Polygenic hypercholesterolemia |
↑ LDL-C |
|
Familial hypertriglyceridemia (200-1,000
mg/dL) |
↑ VLDL-C, ↑ TG |
|
Severe hypertriglyceridemia (≥ 1,000
mg/dL) |
↑ Chylomicrons, ↑ VLDL-C,
↑↑ TG |
|
Familial hypoalphalipoproteinemia |
↓ HDL-C |
|
Dysbetalipoproteinemia (TC: 250-500 mg/dL; TG:
250-600 mg/dL) |
↑ IDL-C, ↑ chylomicron
remnants |
*These are the
two lipid and lipoprotein disorders seen most frequently in childhood and
adolescence; the latter most often manifests with obesity.
HDL-C =
high-density lipoprotein cholesterol; IDL-C = intermediate-density lipoprotein
cholesterol; LDL-C = low-density lipoprotein cholesterol; TC = total
cholesterol; TG = triglyceride; VLDL-C = very-low-density lipoprotein
cholesterol.
Disorders Affecting LDL Receptors
There are five known genetic disorders causing
elevated LDL–C that are expressed in children and that cause early
atherosclerosis and premature CVD; they include FH, FDB, autosomal recessive
hypercholesterolemia, sitosterolemia, and mutations in proprotein convertase
subtilisin-like kexin type 9. These disorders arise from either gene mutations
that affect LDL receptor activity or abnormalities in the LDL receptor itself.
The presence of these disorders indicates a significantly elevated risk for
premature atherosclerosis and CVD events in adulthood.[16] Of
these genetic disorders affecting LDL receptor activity, only FH occurs
commonly enough to be a concern for pediatric care providers.
FH is an autosomal dominant disorder that causes
isolated LDL–C elevation due to gene mutations in the LDL receptor.
Homozygous FH (hoFH) is very rare, with a prevalence of approximately 1:1
million children and is associated with extremely high LDL–C levels (four
to eight times higher than normal). Children with hoFH usually develop CVD by
the second decade of life.[16] Heterozygous FH has a prevalence of
approximately 1:500 children in the United States. In families with known FH,
children with LDL–C levels above 160 mg/dL are likely to have FH.[16] Untreated FH is associated with
premature atherosclerosis and CVD events, with 25 percent of females and 50
percent of males experiencing clinical CVD by age 50 years.
Combined Dyslipidemia
Multiple phenotypes of VLDL–C overproduction and
associated TG and LDL–C elevations have been described. These include
FCHL, familial dyslipidemic hypertension, hyperapoB, and LDL subclass pattern
B. VLDL–C overproduction presents with the lipid pattern of normal to
modest elevation of TC and LDL–C, moderate to moderately severe elevation
of TG, and reduced HDL–C, with increased numbers of small, dense
LDL–C particles.[16] Roughly 20–30 percent of obese
children have evidence of this dyslipidemic pattern.[64],[65],[66],[67],[68],[69]
Since publication of the 1992 NCEP Pediatric Guidelines, the presence
of elevated TG-rich remnants, often reflected as elevated total TG or
non-HDL–C, has become a recognized risk factor for CVD.[70]
From the standpoint of lipoprotein metabolism,
elevated TG in the fasting state most often reflects increased levels of
VLDL–C production from the liver as a consequence of metabolic alterations
associated with obesity. As the TG in VLDL–C are hydrolyzed by lipoprotein
lipase (LPL) and its cofactor apolipoprotein C–II (apoC–II), a series
of VLDL–C remnants of different sizes is produced, ending with IDL–C.
IDL–C can be removed directly from plasma by the interaction of apoE with
the LDL receptor, or the TG on IDL–C can be hydrolyzed by LPL and hepatic
lipase (HL), producing LDL–C. Elevated IDL–C may promote
atherosclerosis by its conversion to LDL–C. As well, IDL–C can be
small enough to cross the endothelial barrier and enter the vascular wall,
where its cholesterol component is atherogenic. In the nonfasting state,
patients with elevated VLDL–C often have delayed removal of TG-enriched
chylomicrons and chylomicron remnants because both VLDL–C and chylomicrons
are competing for LPL. This further accentuates postprandial
hypertriglyceridemia.[71] Finally, elevated TG levels
are usually accompanied by low HDL–C levels, further providing an
atherosclerotic milieu, and are commonly associated with nonlipid risk factors,
such as obesity, hypertension, insulin resistance, and enhanced
thrombogenesis.[16],[67]
Elevated TG may be due to enhanced production of
VLDL–C, decreased hydrolysis, or a combination of both. The most common
cause of elevated TG is increased VLDL–C synthesis. This leads to an
enhanced transfer of TG from VLDL to both LDL and HDL in exchange for
cholesteryl esters (CEs) via the CE transfer protein. As the TG on LDL–C
and HDL–C are hydrolyzed, smaller cholesterol-depleted particles are
produced. Overproduction of VLDL–C leads to an increased number of
atherogenic small, dense LDL–C particles and low HDL–C. Small
HDL–C particles are more avidly removed by the kidney, reducing the number
of HDL–C particles available for reverse cholesterol transport. Increased
VLDL–C production most often results from enhanced hepatic uptake of free
fatty acid (FFA) from plasma, leading to overproduction of TG and apoB. The
elevated FFA is derived from adipose tissue due to insulin resistance and the
decreased inhibition of hormone-sensitive lipase by insulin.
High TG in combination with elevated LDL–C and
reduced HDL–C is the dyslipidemia seen as one of the components of the
metabolic syndrome.[72] Elevated non-HDL–C also
will be present in this dyslipidemic phenotype. The presence of this cluster of
findings in childhood predicts the development of type 2 diabetes mellitus
(T2DM), the metabolic syndrome, and premature clinical CVD in adulthood.[17],[18]
The pediatric aspects of the metabolic syndrome cluster are addressed
separately later in Section XII. Risk Factor Clustering and the Metabolic
Syndrome.
No single gene defect has been identified with the
combined dyslipidemia disorders, which appear to be oligogenic in origin, with
expression exacerbated due to lifestyle factors, especially obesity. In
pediatric lipid clinics to which children are referred because of dyslipidemia,
combined hyperlipidemia is seen about three times as often as FH and is usually
associated with obesity.[73] In families identified because
of an adult proband with clinical CVD and a lipid abnormality (types IIa, IIb,
IV), the expression of combined hyperlipidemia often is delayed until the third
decade of life. However, combined hyperlipidemia appears to be expressed in
adolescents as an elevated apoB level.[29] A recent report
from the longitudinal Young Finns study revealed that, at 21-year followup,
subjects with the combined dyslipidemia pattern beginning in childhood had
significantly increased cIMT compared with normolipidemic controls, even after
adjustment for other risk factors; cIMT was further increased when the
dyslipidemia occurred in the context of the metabolic syndrome.[9]
Given the association with obesity, combined
dyslipidemia is an increasingly common problem.[65] In a
recent study of overweight children, TG levels were significantly elevated in
18 percent of boys and 29 percent of girls, with the degree of elevation
directly correlated with the severity of insulin resistance.[74],[75] The combined
dyslipidemia pattern is now the most common form of dyslipidemia seen in
childhood, and in longitudinal studies, it has been shown to persist into
adulthood.[67],[76]
Normal values for TG are <100 mg/dL in children younger than age 10 years
and <130 mg/dL at ages 10–18 years (see Table 9–1). Obesity and
insulin resistance are usually associated with TG levels between 100 and 400
mg/dL.[77] TG values >500 mg/dL
usually identify an underlying rare genetic abnormality and are addressed
below. Acute conditions associated with severe inflammation and/or endothelial
injury and chronic conditions, such as human immunodeficiency virus (HIV)
infection and cancer chemotherapy, can be associated with marked TG elevation.
Profound hypertriglyceridemia also may occur transiently with ketoacidosis in
type 1 diabetes mellitus (T1DM).
Severe Hypertriglyceridemia
Severe elevation in TG to ≥500 mg/dL is rare in
childhood and is usually associated with genetically based recessive metabolic
defects, including defects in LPL and apoC–II. Severe elevations in TG to
>1,000 mg/dL are associated with increased risk for pancreatitis. With LPL
and apoC–II deficiency, massive increases in chylomicrons and VLDL–C
can occur, producing TG ≥1,000 mg/dL and as high as 5,000–10,000
mg/dL. Such profound increases in TG can produce pancreatitis and eruptive
xanthomas but are not associated with premature atherosclerosis because the
TG-enriched particles are too large to enter the vascular wall. Finally, TG
≥500 mg/dL can be seen in HL deficiency.[16] In
this condition, HDL–C levels are actually elevated. However, so are the
TG-enriched remnants, and premature atherosclerosis can occur in adulthood.
A fasting TG level of ≥ 500 mg/dL often
indicates postprandial elevations to >1,000 mg/dL; children with this degree
of hypertriglyceridemia present a special clinical problem that requires
treatment by a lipid specialist to prevent pancreatitis. These children require
a very low-fat diet (<10 percent fat) undertaken with a nutritionist to
ensure adequate intake of essential fatty acids. Medium-chain TG, which are
absorbed directly into the portal system and do not require chylomicrons for
transport to the liver, can have a significant effect on TG, especially in the
LPL defect. Neither LPL nor apoC–II deficiency responds to lipid-altering
medications. Patients with HL deficiency will respond to lipid-lowering
medication; this is addressed below in the subsection about pharmacologic
therapy.
Low HDL–C Disorders
HDL–C varies inversely with the risk for CVD, and
low HDL–C is an independent predictor of increased risk. In childhood, low
HDL–C is usually expressed as part of combined dyslipidemia accompanied by
obesity, as described previously. It can also be reduced significantly due to
the presence of sedentary lifestyle, cigarette smoke exposure, inherited
defects of low HDL–C production, or increased catabolism. Rare genetic
forms of low HDL–C include familial hypoalphalipoproteinemia, apoA–1
mutations, Tangier disease, and lecithin cholesterol acyltransferase
deficiency. Some, but not all, forms of low HDL–C disorders are associated
with premature CVD.[16]
OVERVIEW OF THE EVIDENCE FOR SCREENING FOR LIPID
DISORDERS IN CHILDHOOD AND ADOLESCENCE
Screening for dyslipidemia in childhood is based on
the concept that early identification and control of dyslipidemia throughout
youth and into adulthood will substantially reduce clinical CVD risk beginning
in young adult life. The primary objectives of screening for dyslipidemia are
the identification of children and adolescents who are (1) at the highest risk
for premature CVD because of extreme lipid abnormalities secondary to inherited
or acquired cholesterol disorders and (2) at increased risk because of
dyslipidemia that is often associated with other risk factors, such as a family
history of CVD or obesity. As described previously, the evidence that children
with dyslipidemia are at significant risk for becoming adults with dyslipidemia
with an increased risk for early CVD is strong. Accurate identification allows
early treatment efforts to focus on children and adolescents at defined risk
for accelerated atherosclerosis.
In 2007, the U.S. Preventive Services Task Force
(USPSTF) published a major systematic review on screening and treatment for
lipid disorders in children.[62] The review noted that in the
included studies, family history questions were not standardized and had
limited diagnostic accuracy. In addition, with the dispersion of families,
knowledge of family history for medical problems was often incomplete. The
reviewers concluded that the evidence demonstrated that using family history as
a primary factor to identify children for screening would miss the majority of
children with inherited dyslipidemias, including approximately 50 percent of
those with FH. Although overweight has been shown to be the best of the known
risk factors for predicting combined dyslipidemia, the review concluded that
the use of other risk factors, alone or in combination, had not been evaluated
adequately to assess their ability to identify children with dyslipidemia. The
review noted that currently recommended screening strategies had low adherence
rates by pediatric health care providers and parents of children at risk. In
addition, studies did not adequately identify the optimal age and frequency of
testing.[62]
In Section IV. Family History of Early Atherosclerotic
Cardiovascular Disease of these Guidelines, a positive family history of early
CVD was identified as important information implying increased risk for future
CVD in offspring. In the previous NCEP Pediatric Panel guidelines, family
history of early CVD was used as the screening tool to define the need for
lipid assessment.[1] Since that time, a number of
studies have evaluated the limitations of this approach. There is no
standardized methodology to assess the family history of CVD, and family
histories are often inaccurate and/or incomplete. Using the recommendations
from the NCEP Pediatric Panel,[1] the proportion of children who have a
family history of premature CVD that will support lipid screening is between 25
and 55 percent. Those studies that used a family history measure to screen for
elevated TC levels found that this method for screening misses between 30 and
60 percent of children with high TC levels. From the Bogalusa study, when a
positive family history of premature CVD was present, there was a higher risk
that the progeny would have abnormal LDL–C levels, but the additional
sensitivity gained was minimal.[78],[79]
Late in adolescence, children with a family history of CVD have been shown to
have higher TC, LDL–C, TG, and blood glucose levels and higher body
weight.[80] However, a negative
family history does not rule out dyslipidemia in children. As noted in the
USPSTF systematic review, although a positive family history of early coronary
heart disease has been shown to predict increased risk for future CVD,
inaccurate and incomplete family history reporting make it neither sensitive
nor specific enough to use as a predictive screening tool for childhood
dyslipidemia.[62] Overweight in children is
associated with significant adverse effects on risk factors, primarily combined
dyslipidemia with elevated TG and low HDL–C levels; these abnormalities
track into adulthood.[67],[76]
Although overweight is the risk factor most predictive of dyslipidemia, the
magnitude of the effect is variable.[64],[65],[66],[67]
In the past, fasting TC levels have been chosen as the
initial screening test by most health care organizations and guidelines. Using
the 95th percentile as abnormal, TC levels in the LRC study have 69 percent
sensitivity and 98 percent specificity in accurately assessing LDL–C
elevations.[47] Using NHANES data, TC levels
had 50 percent sensitivity and 90 percent specificity in detecting elevated
LDL–C levels. As described previously, correlations for TC, LDL–C,
and HDL–C levels with future measures range from approximately 0.4 to
0.6.[62] Approximately half of those
with TC levels above the 75th percentile in childhood will have elevated TC
levels in adulthood.
The issue of appropriate cutoffs for children screened
for lipid disorders was addressed in analyses by both the NCEP Pediatric
Panel[1] and the NHANES III.[48]
In a more recent analysis,[53] TC, LDL–C,
HDL–C, and TG levels from more than 1,700 participants in three
population-based prospective cohort studies were used to compare the ability of
single NCEP cut points with multiple NHANES cut points in adolescence to
predict abnormal levels in adulthood. NCEP cut points were found to be more
predictive of adult high TC, LDL–C, and TG levels than NHANES results but
were less predictive of low HDL–C levels. The likelihood of an adult
having abnormal lipids was significantly higher in those adolescents with
borderline or high lipoprotein levels compared with those with normal levels,
and the increase in risk for adult levels was directly correlated and graded
according to adolescent levels. Acceptable, borderline, and elevated lipid
levels in childhood and young adulthood are shown in Tables 9–1 and
9–2.
Race and gender have both been shown to affect lipid
results. Analysis of more than 4,000 children and adolescents from the Bogalusa
study revealed that after controlling for overweight, White males had
significant adverse changes in TC, LDL–C, VLDL–C, and HDL–C
levels on entering adulthood, with less significant changes for White and Black
females and Black males.[52] By age 26 years, 9 percent of
White males, 8 percent of White females, 2 percent of Black males, and 6
percent of Black females had abnormal lipid profiles, with White males having a
dramatic worsening of the TC/HDL–C ratio.[52] Also from the Bogalusa study,
there were racial differences in TG and VLDL–C levels between Blacks and
Whites, with higher VLDL–C and TG levels in Whites and a modest difference
related to higher HDL–C levels in Blacks.[45],[81]
White female children and Black males had higher HDL–C levels than
White males, although the absolute differences are modest.[45],[81]
Differing distributions of individual risk factors in different groups is
not in itself a reason for different standards for evaluation and/or
management. Race and/or ethnic group-specific recommendations would be
indicated only if there were evidence of a different relationship between risk
factor level and future risk of CVD. At this time, there is insufficient
evidence linking lipid levels to atherosclerosis by race or ethnic group, so
similar cut points are recommended for determining risk status.
The number of children with dyslipidemia continues to
increase along with population increases in overweight and decreases in insulin
sensitivity. Cardiovascular risk factors cluster in children and are strongly
correlated with body fatness.[82],[83]
Childhood overweight is clearly correlated with abnormal lipid levels.[69],[77],[83],[84],[85] Other
conditions—such as diabetes, nephrotic syndrome, chronic renal disease,
inflammatory disease, hypothyroidism, and other secondary causes of
dyslipidemia, known to be associated with accelerated
atherosclerosis—should indicate a higher frequency of testing (see Table
9–3 and Section XI. Diabetes Mellitus and Other Conditions Predisposing to
the Development of Accelerated Atherosclerosis).[86]
Children with these conditions need to be evaluated for dyslipidemia when the
diagnosis of the primary condition is made.
As described previously, non-HDL–C is now
increasingly used in evaluating adults for dyslipidemia. In analyses of two
large pediatric cohorts from the Bogalusa study, non-HDL–C was shown to be
both sensitive and specific for identifying those who will have elevated
LDL–C levels and other dyslipidemias as adults. Children in the top
quartile of non-HDL–C were approximately four times more likely to have
dyslipidemia as adults.[15],[32] A
non-HDL–C level above the 95th percentile was 86–96 percent sensitive
and 96–98 percent specific for detecting an elevated LDL–C level in
both African American and Hispanic children.[62] In a separate
study, the top quartile of non-HDL–C levels correlated with the top decile
of cIMT, as well as did any other lipoprotein measure.[15]
Non-HDL–C levels appear to be a sensitive test for screening, with the
additional advantage of being readily available in the nonfasting state.[32]
As with TC and LDL–C, levels at which risk are identified could be defined
by the 75th and 95th percentiles, as shown in Tables 9–1 and 9–2. A
recent observational study found that non-HDL–C was as powerful as any
other lipoprotein measure for predicting the presence of atherosclerosis in
children and adolescents.[15] For both
children and adults, non-HDL–C levels appear to be more predictive of
persistent dyslipidemia, and therefore atherosclerosis and future events, than
TC, LDL–C, or HDL–C levels alone.[32]
Risks/Harms Associated With Lipid Screening
No studies have identified any consistent harm from
screening for cholesterol in children and adolescents. A concern is whether
screening abnormalities may cause labeling of children, although the evidence
is not sufficient to demonstrate any adverse effects. Although one small
nonrandomized study showed some possible behavior changes in children
identified with dyslipidemia, this has not been substantiated in any of the
many other screening studies, observational trials, or clinical trials.[62]
There is a significant rate of lack of compliance with
screening and followup recommendations by both clinicians and parents of
children with abnormal levels. A number of factors have been suggested,
including inconvenience, discomfort with the screening tests, refusal by the
child or parent, concerns about upsetting the child, resistance regarding
dietary and lifestyle changes, and other unidentified factors.
CONCLUSIONS AND GRADING OF THE EVIDENCE REVIEW FOR
LIPID ASSESSMENT IN CHILDHOOD AND ADOLESCENCE
- Combined evidence from autopsy studies, vascular
studies, and cohort studies strongly indicates that abnormal lipid levels in
childhood are associated with increased evidence of atherosclerosis (Grade
B).
- The evidence review supports the concept that early
identification and control of dyslipidemia throughout youth and into adulthood
will substantially reduce clinical CVD risk beginning in young adult life.
Preliminary evidence in children with heterozygous FH with markedly elevated
LDL–C indicates that earlier treatment is associated with reduced
subclinical evidence of atherosclerosis (Grade B).
- Multiple prospective screening cohort studies have
demonstrated the normal lipid and lipoprotein distributions in childhood,
adolescence, and young adult life (Tables 9–1 and 9–2) (Grade B).
Cohort studies have also demonstrated significant tracking of elevated lipid
levels from childhood to adulthood, with lipid and lipoprotein results in
childhood predictive of future adult lipoprotein profiles; the strongest
statistical correlation occurs between results in late childhood and the third
and fourth decades of life (Grade B).
- TC and LDL–C levels fall as much as10–20
percent or more during puberty (Grade B). Based on this normal pattern of
change in lipid and lipoprotein levels with growth and maturation, age 10 years
(age range 9–11 years) is a stable time for lipid assessment in children
(Grade D). For most children, this age range will precede onset of
puberty.
- Significant evidence exists that using family
history of premature CVD or of cholesterol disorders as the primary factor in
determining lipid screening for children misses approximately 30–60
percent of children with dyslipidemias, and accurate and reliable measures of
family history are not available (Grade B). In the absence of a clinical or
historic marker, identification of children with lipid disorders that
predispose them to accelerated atherosclerosis requires universal lipid
assessment (Grade D).
- Non-HDL–C has been identified as a significant
predictor of the presence of atherosclerosis, as powerful as any other
lipoprotein cholesterol measure in children and adolescents. For both children
and adults, non-HDL–C appears to be more predictive of persistent
dyslipidemia, and therefore atherosclerosis and future events, than TC,
LDL–C, or HDL–C alone. A major advantage of non-HDL–C is that it
can be accurately calculated in a nonfasting state and is therefore very
practical to obtain in clinical practice. The evidence supports use of
non-HDL–C as a screening tool for identification of a dyslipidemic state
in childhood (Grade B).
- In terms of other lipid measurements: (1) at
this time, most but not all studies indicate that measurement of apoB and
apoA–1 for universal screening provides no additional advantage over
measuring non-HDL–C, LDL–C, and HDL–C; (2) measurement of Lp(a)
is useful in the assessment of children with both hemorrhagic and ischemic
stroke; (3) in offspring of a parent with premature CVD and no other
identifiable risk factors, elevations of apoB, apoA–1, and Lp(a) have been
noted; and (4) measurement of lipoprotein subclasses and their sizes by
advanced lipoprotein testing has not been shown to have sufficient clinical
utility in children at this time (Grade B).
- Obesity is commonly associated with a combined
dyslipidemia pattern, with mild elevations in TC and LDL–C, moderate to
severe elevation in TG, and low HDL–C. This is the most common
dyslipidemic pattern seen in childhood, and lipid assessment of overweight and
obese children identifies an important proportion with significant lipid
abnormalities (Grade B).
- Dyslipidemias can be acquired genetically but also
secondary to specific conditions, such as diabetes, nephrotic syndrome, chronic
renal disease, postorthotopic heart transplant, history of Kawasaki disease
with persistent coronary involvement, chronic inflammatory disease,
hypothyroidism, and other causes, as outlined in Table 9–3. There is
impressive evidence for accelerated atherosclerosis both clinically and as
assessed with noninvasive methods in some of these conditions, which
accordingly have been designated as special risk diagnoses for accelerated
atherosclerosis (Table 9–7); management of these is described in Section
XI. Diabetes Mellitus and Other Conditions Predisposing to the Development of
Accelerated Atherosclerosis. Lipid evaluation of these patients contributes to
risk assessment and identifies an important proportion with dyslipidemia (Grade
B).
- The complete phenotypic expression of some
inherited disorders, such as FCHL, may be delayed until adulthood. Evaluation
in children and adolescents from high-risk families with FCHL that begins in
childhood and continues through adulthood (per NCEP adult treatment guidelines)
will lead to early detection of those with abnormalities (Grade B).
Age-specific recommendations for lipid assessment are
outlined in Table 9–5. Specific management for children with identified
dyslipidemia is outlined in the algorithms in Figures 9–1 and 9–2.
Definitions of the risk factors and special risk conditions for use with the
recommendations and in the algorithms appear in Tables 9–6 and 9–7.
The advantages of identifying dyslipidemia and initiating treatment in
childhood are the potential for increased reversibility or slowing of the
disease process, the knowledge that lifestyle change and attention to risk are
more readily accomplished than with individuals in their twenties and thirties,
and the fact that regular contact with the health care system is routine in
this age group. Late adolescence is often the last time for many years that
young adults will routinely undergo health assessment, at the precollege or
preemployment physical. It therefore represents an opportunity to diagnose
lipid disorders and to advise the young adult about his or her CV risk profile
and a healthy lifestyle pattern. When medication is recommended, the decision
occurs in the context of the complete CV risk profile of the patient and the
sociocultural milieu of the family.
The first step proposed for management of children
with identified lipid abnormalities is a focused intervention to improve diet
and physical activity. Conclusions of the evidence review and recommendations
for dietary management of dyslipidemias are provided in the next
subsection.
Table 9–5. Evidence-Based Recommendations for
Lipid Assessment
Grades reflect the findings of the
evidence review.
Recommendation levels reflect the
consensus opinion of the Expert Panel.
NOTE: Values given are in mg/dL. To
convert to SI units, divide the results for total cholesterol (TC), low-density
lipoprotein cholesterol (LDL–C), high-density lipoprotein cholesterol
(HDL–C), and non-HDL–C by 38.6; for triglycerides (TG), divide by
88.6.
| Birth–2 years |
No lipid
screening |
Grade C
Recommend |
| 2–8 years |
No routine
lipid screening |
Grade
B
Recommend |
| 2–8 years (cont.d) |
Measure
fasting lipid profile (FLP) × 2a;
average resultsb if:
- Parent, grandparent, aunt/uncle, or sibling
with myocardial infarction (MI), angina, stroke, coronary artery bypass graft
(CABG)/stent/angioplasty at <55 years in males, <65 years in females
|
Grade
B
Strongly recommend |
| 2–8 years (cont.d) |
- Parent with TC ≥240 mg/dL or known
dyslipidemia
|
Grade
B
Strongly recommend |
| 2–8 years (cont.d) |
- Child has diabetes, hypertension, BMI
≥95th percentile or smokes cigarettes
|
Grade
B
Strongly recommend |
| 2–8 years (cont.d) |
- Child has a moderate- or high-risk medical
condition (Table 9-7)
|
Grade
B
Strongly recommend |
| 9-11 years |
Universal Screening
- Non-FLP: Calculate non-HDL-C:
Non HDL C
= TC - HDL Cc
Non-HDL ≥145 mg/dL,
HDL< 40 mg/dL
→FLP × 2, lipid algorithms belowd
OR
- FLP: LDL-C ≥130 mg/dL, non-HDL-C ≥145
mg/dL
HDL-C <40 mg/dL, TG ≥100 mg/dL if < 10 years; ≥130 mg/dL
if ≥10 years → Repeat FLP after 2 weeks but within 3 months →
lipid algorithms belowd
|
Grade
B
Strongly recommend |
| 12-16 years |
No routine
screeninge |
Grade B
Recommend |
| 12-16 years (cont.d) |
Measure
FLP × 2f, average results, if new
knowledge of:
- Parent, grandparent, aunt/uncle or sibling
with MI, angina, stroke, CABG/ stent/angioplasty, sudden death at < 55 years
in males, < 65 years in females
|
Grade B
Strongly recommend |
| 12-16 years (cont.d) |
- Parent with TC ≥240 mg/dL or known
dyslipidemia
|
Grade B
Strongly recommend |
| 12-16 years (cont.d) |
- Patient has diabetes, hypertension, BMI
≥85th percentile or smokes cigarettes
|
Grade B
Strongly recommend |
| 12-16 years (cont.d) |
- Patient has a moderate- or high-risk medical
condition (Table 9–7)
|
Grade B
Strongly recommend |
| 17-21 years |
Universal
screening once in this time period:
Non-FLP: Calculate
non-HDL–C:
Non-HDL–C = TC – HDL–Cg
17–19
years:
Non-HDL–C ≥145 mg/dL, HDL–C<40
mg/dL
→FLP × 2, lipid algorithm below (Figure 9–1)
OR
FLP:
LDL–C ≥130 mg/dL,
non-HDL–C ≥145 mg/dL
HDL–C < 40 mg/dL, TG ≥130
mg/dL → Repeat FLP after 2 weeks but within 3 months→ lipid
algorithms in Figures 9–1 and 9–2.
20–21
years:
Non-HDL–C ≥190 mg/dL, HDL–C < 40
mg/dLh
→ FLP × 2i average results → Adult Treatment
Panel III (ATP III) management algorithm
OR
FLP:
LDL–C ≥160 mg/dL, non-HDL–C ≥190 mg/dL
HDL–C
<40 mg/dL, TG ≥150 mg/dL → Repeat FLP after 2 weeks but within 3
months, average results → ATP III management algorithm |
Grade B
Recommend |
a Interval
between FLP measurements: after 2 weeks but within 3 months.
b Use Table 9-1 for interpretation of
results; use lipid algorithms in Figures 9-1 and 9-2 for management of
results.
c Disregard TG and LDL-C in
nonfasting sample.
d Use Table 9-1
for interpretation of results; use lipid algorithms in Figures 9-1 and 9-2 for
management of results.
e Lipid
screening is not recommended for those ages 12–16 years because of
significantly decreased sensitivity and specificity for predicting adult
LDL–C levels and significantly increased false-negative results in this
age group. Selective screening is recommended for those with the clinical
indications outlined.
f Interval
between FLP measurements: after 2 weeks but within 3 months.
g Use Table 9-1 for interpretation of
results of 7- to 19-year-olds and lipid algorithms in Figures 9-1 and 9-2 for
management. Use Table 9-2 for interpretation of results of 20- to 21-year-olds
and ATP III algorithms for management
h Disregard TG and LDL-C in nonfasting
sample.
i Interval between FLP
measurements: after 2 weeks but within 3 months
Table 9–6. Risk Factor (RF) Definitions for
Dyslipidemia Algorithms
(+) Family history: myocardial
infarction, angina, coronary artery bypass graft/stent/angioplasty, sudden
cardiac death in parent, grandparent, aunt, or uncle, male <55 years, female
<65 years
High-Level RFs:
- Hypertension requiring drug therapy (BP ≥
99th percentile (%ile) + 5 mmHg)
- Current cigarette smoker
- BMI ≥ 97th %ile
- Presence of high-risk conditions (Table 9-7)
(Diabetes mellitus [DM] is also a high-level risk
factor but it is classified here as a high-risk condition to correspond with
Adult Treatment Panel III recommendations for adults that DM is
considered a CVD equivalent.)
Moderate-Level RFs:
- Hypertension not requiring drug therapy
- BMI ≥ 95th %ile, < 97th %ile "
- HDL-C < 40 mg/dL
- Presence of moderate-risk conditions (Table 9-7)
Table 9–7. Special Risk Conditions
High Risk:
- Diabetes mellitus, type 1 and type 2
- Chronic kidney disease/end-stage renal disease/post
renal transplant
- Postorthotopic heart transplant
- Kawasaki disease with current aneurysms
Moderate Risk:
- Kawasaki disease with regressed coronary aneurysms
- Chronic inflammatory disease (systemic lupus
erythematosus, juvenile rheumatoid arthritis)
- Human immunodeficiency virus infection
- Nephrotic syndrome
OVERVIEW OF THE EVIDENCE FOR DIETARY TREATMENT OF
DYSLIPIDEMIA
BACKGROUND
In the first NCEP guidelines addressing lipids in
children published in 1992, the NCEP Pediatric Panel recommended a prudent diet
(the NCEP Step I diet), with no more than 30 percent of calories from fat, less
than 10 percent of calories from saturated fat, and cholesterol intake less
than 300 milligrams per day (mg/d) for all healthy U.S. children older than age
2 years.[1] Children with dyslipidemias,
primarily those with elevated LDL–C levels, were to be treated first with
the Step I diet; then, if after 3 months they failed to achieve therapeutic
goals, with a more stringent diet (NCEP Step II diet). The NCEP Step II diet
recommended no more than 30 percent of calories from fat, less than 7 percent
of calories from saturated fat, and less than 200 mg/d of dietary cholesterol.
Calories were to be sufficient to maintain normal growth and development. These
recommendations were based primarily on epidemiologic and clinical studies. At
that time, few RCTs addressed the effects of diet modification in children,
particularly during infancy and adolescence, the periods of most rapid growth
and development. The increasing prevalence of obesity in childhood has led to a
large population of children with combined dyslipidemia who also need dietary
management. The evidence review for these Guidelines identified a large number
of observational studies and RCTs that, when combined, provide a substantial
body of information on which to base new recommendations.
EVIDENCE FOR DIETARY TREATMENT OF
HYPERCHOLESTEROLEMIA BY AGE GROUP
Infant Feeding
A meta-analysis of 37 observational cohort and
cross-sectional studies compared the effect of breast-feeding versus
formula-feeding on TC levels in adolescents and adults.[87] Although the mean TC level was
higher in breast-fed versus formula-fed infants, this difference did not
persist into childhood or adolescence. In adults, the TC levels of those who
were breast fed as infants were lower than in those who were formula fed.
Short-term feeding studies, all RCTs with small sample sizes, varied the fat
and cholesterol contents of infant formula, with subsequent changes in levels
of TC, LDL–C, TG, and HDL–C in infancy; there were no differences in
lipoprotein profiles postweaning.[87],[88],[89],[90]
Infancy Feeding Beyond Weaning
Many of the data on the safety and efficacy of a diet
low in saturated fat and cholesterol starting in infancy come from the Special
Turku Coronary Risk Factor Intervention Project (STRIP), in which 7-month-old
Finnish infants (N = 1,062) were randomized into either a group receiving
intensive counseling from a nutritionist for a diet with total fat at
30–35 percent of calories, a 1:1:1 intake ratio of saturated fatty
acid/monounsaturated fatty acid/polyunsaturated fatty acid (PUFA), cholesterol
<200 mg/d, protein (10–15 percent), and carbohydrate 50–60 percent
or into a group receiving basic health education and no instructions on the use
of fats.[91],[92],[93],[94]
Breastfeeding or formula feeding was advised until age 12 months; after age 12
months, the recommended beverage was fat-free milk supplemented with vegetable
fat to maintain total fat intake at the recommended level until age 2 years.
The children were followed with serial evaluations, including dietary
assessment using 4-day food records, to early adolescence. At baseline, there
was no difference in total fat or saturated fat consumption between the groups.
At the first postrandomization lipid evaluation at age 13 months, the diets of
intervention subjects contained a mean of 26 percent of calories from fat, with
9 percent from saturated fat compared with 28 percent and 13 percent,
respectively, in the diets of control subjects, a significant difference
between groups. This change was associated with significantly lower TC and
LDL–C levels in the intervention group, with no differences in measures of
growth and development.[91],[92]
A short-term study varied the fat content of cow's
milk in toddlers between ages 12 months and 2 years. Increasing the vegetable
fat content increased plasma linoleic acid and alpha linoleic acid
concentrations with no change in long-chain PUFA, arachidonic acid, or
docosahexaenoic acid (DHA).[95]
Infancy to Ages 5, 7, and 11 Years
When assessed at ages 3 and 5 years, the STRIP
intervention group consistently had lower intakes of total fat and cholesterol,
higher ratios of polyunsaturated to saturated fat and unsaturated to saturated
fat, and higher intakes of protein and carbohydrate than the control group.[92] These dietary differences were
associated with significantly lower levels of TC, LDL–C, HDL–C, apoB,
and apoA–1 in the intervention group. There were no differences between
the groups in mean energy intake, relative weight, relative height, or
neurologic development.[93] The dietary differences
between the intervention and control groups were maintained at age 7 years.[94] However, only the boys had
significantly lower levels of TC, LDL–C, apoB, and TG. At age 11 years,
with a 55 percent followup rate, intervention boys and girls again had
significantly lower intake of saturated fat and higher polyunsaturated fat to
saturated fat ratios than controls.20 There were no
differences in weight, BMI, or physical activity. In intervention males, TC
levels were 4.6 percent lower, and LDL–C levels were 9.6 percent lower
than control males, but again, there were no significant lipid differences
between groups for females. Of note, intervention boys had significantly
greater endothelial function, as judged by FMD, than control boys, even after
adjusting for differences in LDL–C levels.[20]
A clinically initiated, home-based, parent-child
autotutorial (PCAT) dietary education program directed at increasing dietary
knowledge and reducing fat consumption and LDL–C levels was assessed in an
RCT of 4- to 10-year-old boys and girls with borderline high or high LDL–C
levels.[96] Intervention families received
either individualized diet counseling or use of tape-recorded nutrition
messages aimed at achieving a total dietary fat of less than 30 percent of
calories, saturated fat less than 10 percent of calories, and cholesterol less
than 300 mg/d; control subjects received usual care. At baseline, cholesterol
intake averaged 156 mg/d in the PCAT tutorial group, 163 mg/d in the dietary
counseling group, and 176 mg/d in the control group. After 3 months, those in
the PCAT and the dietary counseling groups, compared with those in the high
cholesterol control group, had significantly lower intakes of total fat as
percentage of calories (-1.5 percent in PCAT; -1.6 percent in diet counseling;
+0.2 percent in high cholesterol controls) and saturated fat (-0.8 percent in
PCAT; -1.0 percent in dietary counseling; no change in high-cholesterol control
group). Cholesterol intake averaged 133 mg/d in the intervention group and138
mg/d in the dietary counseling group and was essentially unchanged at 183 mg/d
in the usual care control group. Mean LDL–C levels decreased significantly
more in the PCAT intervention group by 10 mg/dL, compared with 4.1 mg/dL in the
dietary counseling group and 3.4 mg/dL in the control group. These results were
maintained at 1-year followup.[97] Another pediatric office-based
nutritional education program also effectively decreased total fat, saturated
fat, and cholesterol intakes, with significant decreases in TC and LDL–C
levels after 16 weeks.[98]
In prepubertal children with FH, a restricted diet
with 23 percent ± 5 percent of energy from total fat, 8 percent ±
2 percent from saturated fat, 5 percent ± 1 percent from polyunsaturated
fat, 8 percent ± 2 percent from monounsaturated fat, 15 percent ±
2 percent from protein, 62 percent ± 5 percent from carbohydrate, and
cholesterol 67 ± 28 mg/1,000 kcal for 1 year lowered TC and LDL–C
levels by 4 percent and 5.5 percent, respectively. HDL–C, TG, apoB,
ferritin, weight for height, and height velocity were unchanged.[99]
The Child and Adolescent Trial for Cardiovascular
Health was a group randomized school trial designed to examine the outcomes of
multilevel and multicomponent health behavior intervention in 56 intervention
and 40 control public schools in California, Louisiana, Minnesota, and Texas.
The trial followed 5,106 initially third-grade students from ethnically diverse
backgrounds.[100] In half of the intervention
schools, there were school food service modifications to lower fat and sodium
contents plus enhanced physical education and classroom health curricula; the
other half received the same intervention plus family education. Compared with
control schools, intervention schools had a significant decrease in total fat,
from 38.9 percent to 31.9 percent of energy in cafeteria lunches, and an
increase in the amount of vigorous physical activity. However, after this 2.5
year intervention, there was no difference between the intervention and control
groups in TC levels, the primary outcome. There was no evidence of any
deleterious effect on growth or development.
Adolescents
The STRIP trial has results to age 14 years, at which
time intervention group children still consumed less total and saturated fats
and more carbohydrates and polyunsaturated fat and had lower TC and LDL–C
levels than children in the control group; the difference between groups was
only significant in males. These results were present at the first evaluation
at age 13 months and sustained throughout the study period.[101] There were no harmful effects
identified on growth, micronutrient intake, development, or neurologic
function.[93],[94],[101],[102]
The Dietary Intervention Study in Children[103] assessed the efficacy and safety of
an intervention to lower dietary intakes of total fat, saturated fat, and
cholesterol in order to lower elevated LDL–C levels (between the 80th and
98th percentiles), starting in prepubertal boys (N = 362) and girls (N = 301)
ages 8–10 years and continuing for 3 years. The children were randomized
to an intervention group or a usual care control group in a six-center clinical
trial. A behavioral-based, nutritionist-tailored intervention was used to
promote a diet similar to the NCEP Step II diet—with 28 percent of
calories from fat, less than 8 percent from saturated fat, less than 9 percent
from polyunsaturated fat, and cholesterol less than 75 mg/1,000 kcal/d, not to
exceed 150 mg/d; the control group received dietary literature. At 3-year
followup, dietary total fat, saturated fat, and cholesterol were decreased
significantly in the intervention group compared with the usual care control
group; this was accompanied by small but significant differences in LDL–C
levels (reduction of 15.4 mg/dL in the intervention group versus reduction of
11.9 mg/dL in the control group). Greater sexual maturation and BMI were found
to increase the normal decrease in LDL–C level during adolescence.[104] There were no differences
between the two groups for multiple safety measures. After 3 years, the
intervention was modified to include a more appropriate approach for
adolescents; the significant differences between the two groups in total fat,
saturated fat, and cholesterol intakes were maintained, with no differences in
any of the safety measures, but LDL–C levels did not differ between the
intervention and control groups at 7-year followup.[104],[105],[106]
Young Adulthood, Ages 18–21 Years
Little information is available for this age group. A
small study in university students with moderate LDL–C elevations showed
that a multisession educational intervention significantly improved knowledge
and attitudes about dietary changes compared with controls, but this was not
associated with any significant decreases in TC and LDL–C levels.[107]
OVERVIEW OF THE EVIDENCE FOR LOWERING TC AND
LDL–C LEVELS WITH DIETARY SUPPLEMENTS
Plant Stanol Esters and Plant Sterol Esters
Background
Under normal conditions, few if any plant stanol
esters and only very small amounts of plant sterol esters are absorbed by the
human intestine. Both of these compounds inhibit the absorption of cholesterol,
either by displacing cholesterol from its mixed micelle or by competing with
cholesterol for high-affinity binding sites on the surfaces of intestinal
cells, leading to decreased cholesterol in chylomicrons and less cholesterol
delivered to the liver, a decrease in the hepatic pool of cholesterol, an
induction of LDL–C receptors, and an ultimate decrease of the LDL–C
level.[16] This is associated with increased
biosynthesis of hepatic cholesterol, which limits the efficacy of these
compounds.
Use of Plant Stanols in the General Population
The effect of replacing dietary fat with plant stanol
ester was investigated in a subset of 81, 6-year-old children from the STRIP
trial. TC and LDL–C levels decreased by 5.4 percent and 7.5 percent,
respectively, in those who consumed a plant stanol-enriched margarine as
replacement for 20 grams per day (g/d) of dietary fat intake compared with
control margarine. There was no effect on HDL–C or TG levels. These
changes were accompanied by decreased cholesterol absorption. Safety was judged
to be excellent.[108] Increasing dietary plant
sterols did not alter cholesterol precursor sterol concentrations in these
children.[109] Presence of the apoE–4
variant did not affect the response to plant stanols in the same group of
6-year-olds.[37] There was no significant
difference in decreased cholesterol absorption between boys (36.3 percent) and
girls (42.0 percent), but the compensatory increase in hepatic cholesterol
synthesis was significantly higher in girls (19.5 percent) than in boys (7.6
percent). This might explain the greater decrease in LDL–C levels in boys
(-9.1 percent) than in girls (-5.8 percent). These plant stanol results were
confirmed in a small group of healthy 2- to 5-year-old U.S. preschool
children.[110]
Plant Sterol Esters and Plant Stanol Esters in
Children With Familial Hypercholesterolemia
Five RCTs of plant sterols and plant stanols have been
performed, primarily in FH prepubertal children ages 2–12 years. In each
of these studies, both stanol and sterol esters lowered TC and LDL–C
levels significantly, with decreased absorption of cholesterol accompanied by
increased cholesterol biosynthesis.[111],[112],[113],[114]
Two separate studies in children with FH assessed the
effect of plant sterols on endothelial function and found that despite
significant decreases in TC and LDL–C levels, there was no improvement in
endothelial function, as judged by FMD.[113],[115] This negative
result was ascribed to a relatively small decrease in LDL–C levels
compared with that achieved with statins, which have been shown to improve FMD
in FH children.
Other Dietary Supplements
Dietary supplementation with garlic,[116] soy
protein,[117] and DHA[118] did not lower LDL–C
levels in hypercholesterolemic children. However, DHA supplementation restored
impaired FMD. In some but not all studies, psyllium significantly lowered
LDL–C levels from 5 to 10 percent, but there was no identification of
dietary sources of fiber, so results are difficult to interpret.[119],[120],[121] In a single study, adding
vitamins C and E to the low-fat diets of FH or FCHL children was associated
with an improvement in FMD, independent of changes in blood lipid and
lipoprotein levels.[122]
OVERVIEW OF THE EVIDENCE FOR DIETARY MANAGEMENT
OF HYPERTRIGLYCERIDEMIA
Elevated TG levels are very responsive to weight loss,
diet composition, and exercise. Most importantly, in overweight and obese
children and adolescents with elevated TG levels, even small amounts of weight
loss are associated with significant decreases in TG levels and increases in
HDL–C levels.[123],[124],[125],[126] Exercise training alone, when
associated with a decrease in body fat, has also been shown to be associated
with a significant decrease in TG levels, with reversion to baseline when
children became less active.[127]
Regarding dietary composition, substitution of soy
milk for low-fat cow's milk induced significant reductions in TG and
VLDL–C levels and increased HDL–C levels in a small series of
children.[128] In adults with
hypertriglyceridemia, a low-carbohydrate, high-fat diet (40 percent
carbohydrate, 39 percent total fat, 8 percent saturated fat, 15 percent
monounsaturated fat) significantly decreased TG by a mean of 63 percent, with
associated mean increases in LDL–C of 22 percent and HDL–C of 8
percent. A subsequent high-carbohydrate, low-fat diet (54 percent carbohydrate,
28 percent total fat, 7 percent saturated fat, 10 percent monounsaturated fat)
significantly increased TG back to baseline levels.[129] In children, a 12-month
followup study of 21-month-old children with elevated TG levels treated with a
carbohydrate-restricted diet showed a decrease in sugar and carbohydrate
intakes associated with a decrease in TG from a mean of 274.1 +/- 13.1 mg/dL
before treatment to 88.8 +/- 13.3 mg/dL after 12 months.[130] In an analysis of adolescents
from NHANES, the U.S. Department of Agriculture's Center for Nutrition Policy
and Promotion's Healthy Eating Index (HEI) was used to provide an overall
picture of dietary quality relative to the metabolic syndrome constellation of
central obesity, elevated TG, elevated BP, reduced HDL–C level, and
impaired fasting glucose level.[131] There was a
significant inverse association between the overall HEI score plus the fruit
intake score and the prevalence of the metabolic syndrome components. There was
also a trend toward lower prevalence of the metabolic syndrome components,
including elevated TG in adolescents with high activity levels, although this
was not significant. The concept of glycemic load has also been evaluated in
the setting of obesity and dyslipidemia in adolescents and adults. The glycemic
index is a measure of the blood glucose response to a 50 g portion of a
selected carbohydrate; the glycemic load is the mathematic product of the
glycemic index and the carbohydrate amount.[132] In
adolescents and young adults, there is evidence that low glycemic-load diets
are at least as effective as low-fat diets in achieving weight loss, with
decreased TG and increased HDL in subjects on the low glycemic-load diet.[133],[134],[135] In adolescents, a
low-carbohydrate diet associated with weight loss has been shown to
significantly reduce TG levels.[136]
CONCLUSIONS AND GRADING OF THE EVIDENCE REVIEW FOR
DIETARY MANAGEMENT OF DYSLIPIDEMIA
- A diet with total fat at 25–30 percent of
calories, saturated fat less than 10 percent of calories, and cholesterol
intake less than 300 mg/d, as recommended by the original NCEP Pediatric Panel,
has been shown to safely and effectively reduce the levels of TC and LDL–C
in healthy children. (Grade A). There is some evidence this is also the case
when the diet begins in infancy and is sustained throughout childhood into
adolescence (Grade B). The Cardiovascular Health Integrated Lifestyle Diet
(CHILD 1), described in Section V. Nutrition and Diet of these Guidelines, has
this composition.
- In children with identified hypercholesterolemia
and elevated LDL–C levels, a more stringent diet, with saturated fat <7
percent of calories and dietary cholesterol limited to 200 mg/d, has been shown
to be safe and modestly effective in lowering LDL–C levels (CHILD 2-LDL,
Table 9-8) (Grade A).
- Use of dietary adjuncts, such as plant sterol or
stanol esters, up to 2 g/d can safely enhance the LDL–C-lowering effects
short term in children with FH (Grade A). However, long-term studies on the
safety and effectiveness of plant sterol and stanol esters have not been
completed. Their clinical use is therefore usually reserved for children with
primary elevations of LDL–C who do not achieve LDL–C goals with
dietary treatment alone. Such an approach may lower LDL–C sufficiently to
avoid the necessity of drug treatment. Food products containing plant stanol
esters, such as some margarines, are marketed directly to the general public.
In two short-term trials, they have been shown to be safe with minimal
LDL-lowering effects in healthy children (Grade B).
- Evidence for use of other dietary supplements is
insufficient for any recommendation (no grade).
- In children with elevated TG, weight loss and
reduction of simple carbohydrate intake are associated with decreased TG
levels. (CHILD 2-TG, Table 9-8) (Grade B). When elevated TG are associated with
obesity, decreased calorie intake and increased activity levels are of
paramount importance.
- A behavioral approach that engages the child and
family delivered by a registered dietitian has been shown to be the most
consistently effective approach for achieving dietary change (Grade B).
The approach to management of dyslipidemias is
staged, as in the original NCEP Pediatric Panel recommendations. For all
children with identified dyslipidemia in whom the response to a low-fat/low
saturated fat/low cholesterol diet has not been evaluated, the CHILD 1 diet
described in Section V. Nutrition and Diet is recommended as the first step,
with implementation guided by a registered dietitian. For obese children with
identified dyslipidemia, age- and BMI-specific additional recommendations
addressing calorie restriction and increased activity appear in Section X.
Overweight and Obesity. If, after a 3-month trial of CHILD 1/lifestyle
management, fasting lipid profile findings exceed the therapeutic goals in
Tables 9-1 and 9-2, lipid parameter-specific diet changes outlined in Table 9-8
are recommended. Dyslipidemia management is also outlined in the algorithms in
Figures 9-1 and 9-2.
Table 9–8. Evidence-Based Recommendations for
Dietary Management of Elevated LDL–C, non-HDL-C and TG
Grades reflect the findings of the
evidence review.
Recommendation levels reflect the
consensus opinion of the Expert Panel.
Supportive actions
represent expert consensus suggestions from the Expert Panel provided to
support implementation of the recommendations; they are not graded.
NOTE: Values given are in mg/dL. To
convert to SI units, divide the results for total cholesterol (TC), low-density
lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C),
and non-HDL-C by 38.6; for triglycerides (TG), divide by 88.6.
ELEVATED LDL–C: CHILD 2 -
LDL
| 2–21 years |
Refer to a registered
dietitian for family medical nutrition therapy: |
Grade B
Strongly
recommend |
| 2–21 years (cont.d) |
- 25–30% of calories from fat, ≤7% from
saturated fat, ~10% from monounsaturated fat; <200 mg/d of cholesterol;
avoid trans fats as much as possible
Supportive actions:
- Plant sterol esters and/or plant stanol
esters* up to 2 g/d as replacement for
usual fat sources can be used after age 2 years in children with familial
hypercholesterolemia.
- Plant stanol esters as part of a regular diet
are marketed directly to the public. Short-term studies show no harmful effects
in healthy children.
- The water-soluble fiber psyllium can be added
to a low-fat, low saturated fat diet as cereal enriched with psyllium at a dose
of 6 g/d for children 2–12 years, and 12 g/d for those ≥12
years.
- As in all children, 1 hour/day (h/d) of
moderate to vigorous physical activity and <2 h/d of sedentary screen time
are recommended.
|
Grade A
Recommend |
* Can be found
added to some foods, such as some Margarins
ELEVATED TG OR NON-HDL–C: CHILD 2 -
TG
| 2–21 years |
Refer to a registered
dietitian for family medical nutrition therapy:* |
Grade B
Strongly
recommend |
| 2–21 years (cont.d) |
- 25–30% of calories from fat , ≤7%
from saturated fat, ~10% from monounsaturated fat; <200 mg/d of cholesterol;
avoid trans fats as much as possible
|
Grade A
Recommend |
| 2–21 years (cont.d) |
- Decrease sugar intake:
- Replace simple with complex
carbohydrates
- No sugar sweetened beverages
|
Grade B
Recommend |
| 2–21 years (cont.d) |
- Increase dietary fish to increase omega-3
fatty acids**
|
Grade D
Recommend |
* If child is
obese, nutrition therapy should include calorie restriction, and increased
activity (beyond that recommended for all children) should be prescribed. See
Section X. Overweight and Obesity for additional age-specific recommendations.
** The Food and Drug Administration
(FDA) and the Environmental Protection Agency are advising women of
childbearing age who may become pregnant, pregnant women, nursing mothers, and
young children to avoid some types of fish and shellfish and eat fish and
shellfish that are low in mercury. For more information, call the FDA's food
information line toll free at 1–888–SAFEFOOD or visit
http://www.cfsan.fda.gov/~dms/admehg3.html.
MEDICATION THERAPY FOR HYPERLIPIDEMIAS
Background
1992 NCEP Recommendations/American Heart Association
Scientific Statement/American Academy of Pediatrics' Committee on Nutrition
Recommendations
In addition to making recommendations for screening
and lifestyle management of LDL abnormalities, the 1992 NCEP Pediatric Panel
report made recommendations regarding medication therapy.[1]
It was recommended that treatment with medications be considered only in
children ages 10 years and older after an adequate 6- to 12-month trial of
lifestyle/dietary modification. The criteria for initiation included LDL–C
levels ≥190 mg/dL or LDL ≥160 mg/dL, together with either a
positive family history of premature CVD or the presence of two or more other
CVD risk factors. The only medication recommended was a bile acid sequestrant.
At that time, no data were available regarding the safety and efficacy of
statin use in children.
Since the NCEP Pediatric Panel guidelines were
published in 1992, new evidence, as described in the opening section, provides
the impetus for their reevaluation and modification. In 2007, the American
Heart Association (AHA) published a scientific statement with updated treatment
recommendations for children and adolescents.[137] The AHA guidelines
recommended that the presence of overweight and obesity was an additional
indication for screening with a fasting lipid profile (FLP) and that the
presence of overweight and lipid abnormalities indicated screening for other
abnormalities associated with insulin resistance/metabolic syndrome. For
patients meeting the criteria for pharmacologic therapy, the AHA recommended a
statin as a first-line agent. The presence of other risk factors and high-risk
conditions should be considered in making decisions regarding medication
therapy, including LDL–C levels and age for beginning treatment and target
LDL–C levels to be achieved. However, the AHA made no specific
recommendation about how these other risk factors and conditions were to be
considered; this was left to the discretion of the treating health care
provider.
More recently, the American Academy of Pediatrics'
Committee on Nutrition published guidelines for the screening and management of
lipid abnormalities in children.[138] In these
recommendations, pharmacologic therapy is primarily considered at or after age
10 years. Rarely, initiation of treatment with medications could be considered
in FH patients ages 8 years and older. Children younger than age 8 years with
extreme elevation of LDL–C levels, above 500 mg/dL (as with hoFH), also
might be considered for pharmacologic therapy. The Committee also recommended
that children with diabetes mellitus and LDL–C ≥130 mg/dL be
considered for drug therapy.
Consideration of Associated Risk Factors/High-Risk
Conditions
Both pathology studies and studies using noninvasive
assessment of vascular markers have shown an exponential increase in arterial
abnormalities with increasing numbers of risk factors. For such patients,
intensification of therapy and revised thresholds for initiation and treatment
targets should be considered, particularly if those other risk factors are
present at a higher magnitude or there is a higher level of individual risk. In
addition, some patients may have high-risk conditions that are associated with
established premature CVD or that serve as additional accelerators to the
atherosclerotic process. These high-risk conditions have been highlighted and
their management discussed in an AHA scientific statement on CV risk reduction
in high-risk pediatric patients.[86] A high-risk
condition was defined as one associated with manifest CAD at younger than age
30 years; high-risk conditions included hoFH, T1DM, chronic or end-stage renal
disease or postrenal transplant, Kawasaki disease complicated by persistent
coronary artery aneurysms/stenoses/occlusions, and patients with orthotopic
heart transplantation. It was recommended that patients with these conditions
be routinely screened for CV risk factors and that those identified be
aggressively managed. Moderate-risk conditions were defined as those associated
with pathophysiologic evidence that the atherosclerotic process was accelerated
and included FH, Kawasaki disease with regressed coronary artery aneurysms,
T2DM, and chronic inflammatory disease (juvenile onset rheumatoid arthritis and
systemic lupus erythematosus). It was recommended that these patients also be
routinely screened for CV risk factors, although levels for initiation of
intervention and therapeutic targets were less aggressive. For these
Guidelines, either T1DM or T2DM is considered a high-risk condition, and HIV
and nephrotic syndrome are added as moderate-risk conditions (Table 9–7).
Specific management of these high-risk conditions is outlined in Section XI.
Diabetes Mellitus and Other Conditions Predisposing to the Development of
Accelerated Atherosclerosis.
OVERVIEW OF THE EVIDENCE FOR SAFETY AND EFFICACY OF
MEDICATION THERAPY
Since the 1992 NCEP Pediatric Panel guidelines, a
series of RCTs of medications to treat lipid abnormalities in children and
adolescents have been completed; all of these are outlined in Table 9–10
and included in the evidence tables that will be available at
http://www.nhlbi.nih.gov/guidelines/cvd_ped/index.htm.
Several clinical trials of statins[21],[22],[23],[139],[140],[141], [142],[143],[144],[145] and
bile acid sequestrants[99],[146],[147],[148] for
the treatment of severe elevations of LDL–C in children with FH have been
completed and are shown in Table 9–11. These studies have been conducted
in children and adolescents with severe dyslipidemia or FH who met the
recommendations of the 1992 NCEP guidelines for initiation of medication
therapy.[1] FH in children was defined as
having a family history of elevated LDL–C, atherosclerosis, or CAD in
conjunction with having elevated LDL–C. The LDL levels for trial
eligibility ranged from a lower limit of 154–189 mg/dL or 95th percentile
for age and gender. Per the 1992 NCEP recommendations, almost all the studies
tested drug therapy after a trial of diet. The studies have been of relatively
short duration, ranging from 6 weeks to 2 years, with several longer than 4
months; one trial was extended as an open-label study to 7.4 years, with both
randomized groups receiving drug therapy. Patients in early puberty have been
included. Trial subjects were monitored carefully throughout the treatment
period. No impact on growth, development, or sexual maturation has been
identified; adverse event profiles and efficacy were similar to those noted in
studies of adults. Because of problems with palatability, compliance with the
bile acid sequestrants has been generally problematic. The details of the
safety and efficacy of the statin medications in children are described below
with the management of FH. The specific LDL–C-lowering effects of each
medication are shown in Table 9–10.
There is limited published experience in children with
use of niacin and fibrates, which may be useful in treating patients with
combined dyslipidemias.[149],[150] Efficacy and safety data are
limited, and no data are available regarding newer formulations. In adults,
cholesterol absorption inhibitors have been advocated as an adjunct to statin
therapy for patients who do not reach LDL–C therapeutic targets. Since
their action is independent of and complementary to that of statins, the
LDL–C-lowering effect is additive. No pediatric studies of monotherapy
with cholesterol absorption inhibitors had been published during the time
period for this evidence review. Use of niacin, fibrates, and
cholesterol-absorption inhibitors should be instituted only in consultation
with a lipid specialist.
OVERVIEW OF THE EVIDENCE OF THE IMPACT OF MEDICATION
THERAPY ON VASCULAR MARKERS
Although it is unlikely that studies will ever
document that treatment of lipid abnormalities in youths will reduce manifest
atherosclerotic disease and CV events when they become adults, there is
emerging evidence using noninvasive vascular markers that lipid-lowering
therapy improves arterial function and structure. From this evidence review,
brachial artery reactivity by ultrasound FMD has been assessed as a measure of
endothelial function in a clinical trial of simvastatin for children and
adolescents with FH.[23] At baseline, both placebo and
statin intervention FH groups had impaired FMD in response to reactive
hyperemia, but this improved significantly in the group treated with
simvastatin for 28 weeks. Carotid intima-media thickness by ultrasound has been
evaluated as a marker of early atherosclerosis in a clinical trial of
pravastatin for children and adolescents with FH.[21]
After 2 years, cIMT had increased in the placebo group, but there was
significant regression in the group treated with pravastatin. An open-label
followup study of these patients for an average of 4.5 years reported that
earlier initiation of statin therapy was associated with smaller cIMT at
followup, after adjusting for baseline cIMT, gender, and duration of
treatment.[22] This study included patients
who had started treatment at age 8 years. These findings contrast with a
nonplacebo-controlled, single-arm study with fluvastatin for 2 years, in which
no significant changes in cIMT or wall stiffness were noted in response to
LDL–C reduction.[140] It is important to note that
the changes observed in these studies occurred despite the fact that patients
did not necessarily achieve recommended LDL–C target cut points and had
important residual elevations in LDL–C. These findings represent early
evidence that therapy with statins in youth may have a significant positive
impact on the atherosclerotic process. These results and findings of better
endothelial function assessed by FMD in adolescent boys with lower LDL from
infancy[20] suggest the potential benefit of
initiation of LDL-lowering treatment in childhood. The atherosclerotic process
is not uniform over a lifetime, and it is probable that early lesions are more
effectively treated and reversed than more advanced lesions; this provides some
additional potential rationale for initiating drug therapy in youth with
severely elevated LDL–C levels.
OVERVIEW OF THE EVIDENCE FOR MANAGEMENT OF SPECIFIC
LIPID ABNORMALITIES
Heterozygous Familial Hypercholesterolemia
Heterozygous FH is associated with markedly elevated
LDL–C levels and with normal or low HDL–C and usually but not always
normal TG levels. FH is inherited as an autosomal dominant trait, with
prevalence in the general population of 1:500 but higher in certain ethnic
groups (e.g., French Canadians, South Africans, Lebanese). More than 500
mutations resulting in abnormalities of the LDL–C receptor have been
identified, ranging from null alleles blocking LDL–C receptor formation
and processing to defects resulting in defective receptors with diminished
functionality. Alternatively, a similar phenotype is noted for patients with
defects of the LDL–C receptor ligand apoB. All these abnormalities result
in impaired LDL–C clearance. FH is associated with acceleration of
atherosclerosis and premature CVD or events, beginning at ages thirties to
forties in men and forties to fifties in women. The risk for clinical events is
influenced by the presence of other risk factors or conditions. It is unclear
whether FH confers an increased risk beyond that associated with the attendant
lipid abnormalities; the fact that the lipid abnormalities are unrelenting from
birth may impart an increased cumulative risk beyond that of other conditions
and acquired abnormalities. In children with FH, LDL–C elevations are such
that the vast majority will meet the criteria for treatment with medication
with statins as the mainstay, as reviewed below and in Table 9–11.[21],[22],[23],[139],[140],[141], [142],[143],[144],[145],[151]
A recent systematic review and meta-analysis of statin
therapy in children with FH analyzed studies that included almost 800
children.[152] No statistically significant
differences were found between statin-treated and placebo-treated children for
the occurrence of adverse events, sexual development, muscle toxicity, or liver
toxicity; there was a minimal difference in growth in favor of the statin
group. The LDL–C level and the timing for introduction of medication
therapy are outlined in the algorithm (see Figure 9–1), and recommended
medications appear in Tables 9–10 and 9–11. Response to the statins
can be variable and may relate to the underlying specific genetic abnormality;
some patients may require additional therapy, such as bile acid sequestrants,
to achieve target LDL–C levels.[99],[146],[147],[148],[153]
Cholesterol absorption inhibitors have also been recommended in this situation.
A recent RCT of pediatric patients ages 10–17 years with FH demonstrated
that coadministration of the cholesterol absorption inhibitor ezetimibe with
simvastatin resulted in significantly greater reductions in LDL–C than did
simvastatin alone; the combination was safe and well-tolerated up to 53
weeks.[154] For those with associated
HDL–C and TG abnormalities, intensification of statin therapy or
additional therapy with fibrates or niacin would be recommended. Any
combination therapy should be undertaken in consultation with a lipid
specialist. At this time, the need for therapy and monitoring is lifelong.
Homozygous Familial Hypercholesterolemia
Homozygous FH results in extreme elevations of
LDL–C levels (often 5–10 times the upper limit of normal) and
decreased HDL–C levels. The magnitude of LDL–C abnormalities may be
influenced by which of the two mutations is inherited; these are often not
concordant. CVD is usually manifest by the second decade, consisting primarily
of coronary ostial stenoses and occlusions, aortic valve thickening with
stenosis and/or regurgitation, and extensive atherosclerosis of the aortic
root. The most common mode of presentation of hoFH is physical manifestations
in infancy and early childhood, consisting of primarily fleshy cutaneous
xanthomata between the fingers and toes and over the buttocks, elbows, and
knees and tendonous xanthomata, most marked in the Achilles tendon, with
nodularity and thickening. Because of the cutaneous manifestations, the
diagnosis is often made by dermatologists; additional investigation and
management should be made by a lipid specialist. A complete cardiologic
investigation is indicated at the time of presentation, since important CVD
already may be present, and ongoing careful monitoring is important. There are
no RCTs of treatment for hoFH. Despite severely reduced LDL–C receptor
capacity, patients may respond somewhat to high doses of statins and to
cholesterol absorption inhibitors.[16],[155] However, the majority of
patients will require artificial clearance of circulating LDL. LDL apheresis
specifically removes LDL and is preferred to plasmapheresis, which depletes HDL
as well as LDL. LDL apheresis usually is performed biweekly in medical centers
with this expertise. At this time, the need for therapy and monitoring is
lifelong. Liver transplantation is no longer recommended for children with hoFH
because of the marked side effect profile of the procedure. The current goal of
therapy is palliation of the disease until a time when effective and safe gene
therapy becomes available.
Severe Primary Hypertriglyceridemias
In children with severe hypertriglyceridemia for whom
diet and exercise interventions are insufficient, there are nutriceutical and
medication options that can be considered. The TG level and the timing for
introduction of more advanced therapy are outlined in the algorithm (see Figure
9–2), and recommended medications are shown in Tables 9–10 and
9–11. A recent systematic review demonstrated that omega-3 fish oil
capsules are both safe and effective in adults, reducing TG by 30–45
percent, with significant associated increases in HDL–C.[156] For children, the safety of
omega-3 fish oil was observed in their use in children with immunoglobulin A
nephropathy and in a small series of children with dyslipidemia.[157] Because fish oil preparations
are marketed directly to the public, pediatric care providers can expect to
encounter children who are taking these supplements. Information about how to
evaluate the various preparations available is provided at the bottom of Table
9–11. In adults, fibrates have been used to lower TG levels, and a small
series in children demonstrated effective reductions in TG levels and an
associated increase in HDL–C levels.[150] Finally, niacin
has been used extensively in adults, but there is limited experience in
children, with a single series demonstrating a high rate of side effects.[149] The use of either fibrates or
niacin in youths should be undertaken only with the assistance of a lipid
specialist.
Children with sustained TG levels ≥500 mg/dL
present a rare and serious clinical problem that is usually associated with an
underlying genetic defect (LPL deficiency, HL deficiency, or apoC–II
deficiency). They are at high risk for pancreatitis beginning in infancy.
Management of these patients should always be in consultation with a lipid
specialist. These children require a very low-fat diet (<10 percent fat)
undertaken with a nutritionist to ensure adequate calories and intake of
essential fatty acids. Medium-chain TG, which are absorbed directly into the
portal system and do not require chylomicrons for transport to the liver, can
have a significant lowering effect on TG levels, especially in those with
defective or deficient LPL. Patients with either LPL or apoC–II deficiency
do not respond to lipid-altering medications, but patients with HL deficiency
will respond to fibrates, niacin, or statins. As indicated in the algorithm,
management of these patients should always be in consultation with a lipid
specialist.
Isolated Low HDL–C Levels
Isolated low HDL–C levels can occur as a primary
abnormality in HDL–C metabolism; this is associated with an increased risk
of premature CVD in affected family members. Currently, it is unclear whether
this condition contributes to accelerated atherosclerosis in youth, there is no
evidence concerning management of this condition in youth, and there is no
evidence that treatments aimed at increasing HDL–C levels are effective
and safe. There has been some beneficial effect on HDL–C levels associated
with niacin therapy.[16],[149]
There are no RCTs of medication therapy for isolated low HDL–C
levels in childhood. Current recommendations for management of this abnormality
include attention to lifestyle modification and to abnormalities in TG,
LDL–C, and non-HDL–C levels, including lower levels at which to
initiate therapy and lower therapeutic target levels. For example, some
patients who present with isolated HDL–C levels can have an elevation in
the number of small, dense LDL particles that can be detected by determining
the apoB level under the direction of a lipid specialist. More aggressive
management of other risk factors/conditions is also indicated in the presence
of isolated low HDL–C levels.
Elevated Lipoprotein(a)
There is currently no medication therapy specific for
elevated Lp(a), and similar to isolated low HDL–C levels, management may
focus on addressing other risk factors and on more aggressively managing
concomitant elevations of LDL–C, TG, and non-HDL–C.
In adults, niacin will lower Lp(a) approximately 15
percent, but this has not been studied in children.
Combined Lipid Abnormalities
The most common combination of lipid abnormalities is
that associated with obesity, the triad of low HDL–C, high TG, and a mild
increase in LDL–C levels, with a qualitative change in LDL molecules such
that the particle is smaller and denser and particle numbers are higher,
contributing to a more atherogenic milieu. In general, these patients do not
meet the criteria for pharmacologic therapy, and management focuses on reducing
adiposity and changing diet composition as described in the previous lipid
subsection on the dyslipidemias. Diet and lifestyle management for elevated TG
levels are outlined in the subsection on the dietary treatment of the
dyslipidemias, the CHILD 2-TG diet (Table 9–8). However, given that some
patients will have clustering of risk factors, there may be a selective role
for pharmacologic therapy, most commonly directed at elevations in
non-HDL–C levels. Treatment with statins, omega-3 fish oil, or fibrates
might be considered, but evidence for this in children is very limited, and
there have been no RCTs.
Several primary dyslipidemias are associated with
elevations in VLDL–C level, which is often manifest as an elevated
LDL–C level together with a high TG level. Specific therapies are not
available, and pharmacologic therapy is usually guided by non-HDL–C
levels. Statins, omega-3 fish oil, or fibrates should be considered as
first-line agents for patients who meet the criteria for medication therapy,
under the direction of a lipid specialist.
Guidelines for Initiating and Monitoring Medication
Therapy
Statins (Hydroxymethylglutaryl Coenzyme A Reductase
Inhibitors)
Given the widespread experience with statins in adult
patients, their greater efficacy and tolerance, and the presence of a number of
well-designed (albeit relatively short-term) studies in children and
adolescents (see Table 9–11),[21],[22],[23],,[140],[141], [142],[143],[144],[145], [151] statin therapy is recommended as
the initial medication of choice for treating patients with sufficiently
elevated LDL–C or non-HDL–C levels (see algorithm in Figure
9–1). The statins inhibit hydroxymethylglutaryl coenzyme A reductase,
which is a rate-limiting enzyme in the endogenous cholesterol synthesis
pathway. This results in a decrease in the intracellular pool of cholesterol,
which signals upregulation of LDL–C receptors and increased clearance of
circulating LDL–C.
The starting dose used for each of the statin
medications in the RCTs is shown in Table 9–11. These are preparation
specific. Use of these medications requires that practitioners become familiar
with the dose recommendations for one of the statins. Statin use should begin
with the lowest available dose given once daily. If LDL–C target levels
are not achieved with at least 3 months of compliant use, then the dose may be
increased by one increment. The risk and effectiveness of dose escalation have
been explored in several of the statin clinical trials in children, with no
additional safety issues identified.
Adverse effects from statins are rare at standard
doses but include myopathy and hepatic enzyme elevation. Asymptomatic hepatic
enzyme elevation is fairly common in adults on statin therapy but is reversible
with medication change and is not clearly associated with increased risk of
liver disease. In the meta-analysis of statin use in children, evidence of
hepatic enzyme elevation did not differ between the statin and placebo
groups.[152] Myopathy—muscle pain and
weakness with creatine kinase elevations more than 10 times the upper limits of
normal range—typically occurs in fewer than 1 in 10,000 adult patients.
Evidence of muscle toxicity did not differ between the statin and placebo
groups in the meta-analysis of statin use in children.[152]
Rhabdomyolisis, a very rare occurrence in adults on statin therapy reported at
3 per 100,000 person-years, did not occur in any of the pediatric trials but
the total number of subjects is too small to evaluate that risk. The risk of
rhabdomyolysis increases with use of higher doses and interacting drugs. Drug
interactions with statins occur primarily with drugs that are metabolized by
the cytochrome P–450 system, the primary mode of metabolism for the
majority of statins. Drugs that potentially interact with statins include
fibrates, azol antifungals, macrolide antibiotics, antiarrhthymics, and
protease inhibitors. When statin use is initiated, prescribing information must
be routinely consulted for potential drug interactions. Patients need to be
cautioned about potential future medication interactions, and pediatric care
providers need to assess this whenever any new medication is introduced.
In addition to significantly lowering LDL–C
levels, statins may increase HDL–C levels modestly and lower TG
modestly—effects that are considered beneficial. In adults, treatment with
statins also decreases inflammation as judged by the lowering of
high-sensitivity C-reactive protein, considered to be a pleiotropic effect of
statins. This test is not established for use in the management of lipid
disorders in children and adolescents. The statins do not influence levels of
the essential fatty acids necessary for early central nervous system maturation
and have not been shown to affect neurodevelopment, although studies in infants
and very young children have not yet been conducted. Clinical trials have
included both male and female children studied over the time of puberty and
have shown no impact on sexual maturation or height velocity. Specific
guidelines for the use of statins are given in Table 9–12.
Bile Acid Sequestrants
Bile acid sequestrants were the initial medication of
choice recommended in the original NCEP Pediatric Guidelines. The
rationale was that these agents were not systemically absorbed and thus were
believed to be safer for children and adolescents. The sequestrants bind bile
salts within the intestinal lumen and prevent their enterohepatic reuptake in
the terminal ileum, resulting in a depletion of bile salts in the liver and a
signal for increased production. Since bile salts are synthesized from
intracellular cholesterol in the liver, the intracellular pool of cholesterol
becomes depleted, signaling increased production of LDL–C receptors and
increased clearance of circulating LDL–C to replenish the intracellular
cholesterol pool for increased production of bile salts. The sequestrants are
available in tablet and powder formulations (to be swallowed with or mixed with
a liquid of preference). Studies of bile acid sequestrants (cholestyramine,
colestipol, colesevelam) in children and adolescents with FH and hence more
extreme elevations of LDL–C levels, show reductions of LDL–C levels
of 10–20 percent and sometimes a modest elevation in TG levels (see Table
9–11).[99],[147],[148],[153]
The primary adverse effects of the bile acid sequestrants tend to be
gastrointestinal in nature, including bloating, nausea, diarrhea, and
constipation; these significantly affect compliance. Since the bile acid
sequestrants reduce bile salts, which are important for intestinal lipid
absorption, there has been some concern regarding malabsorption of fat-soluble
vitamins (A, D, E),[99][158] and routine
supplementation with a daily multivitamin and folate may be indicated. Also,
bile acid sequestrants may interfere with the absorption of some medications;
this potential interaction should be specifically evaluated whenever any
additional medication is needed.
The efficacy and adverse effects of bile acid
sequestrants are somewhat dose dependent. The degree of LDL–C reduction is
often insufficient to achieve LDL–C target levels for the majority of
those patients who meet the criteria for pharmacologic therapy, and tolerance
and compliance have been reported to be variable in children and may be
influenced by the formulation used.[99][147],[148] The
initial tablet dose (colestipol) is five tablets (5 g)/d and for the powder
(cholestyramine) one packet (4 g)/d. The dose can be titrated upward according
to tolerance to a maximum of 20 g/d. The dosage can be divided throughout the
day according to the patient's preferences and can be taken with meals. Tablets
should not be chewed or divided. Colesevelam is in tablet form with starting
dose of 1.875 g/day; dose can be up-titrated to 3.75 g/day; in the clinical
trial, compliance with colesevelam was satisfactory. Since the formulation can
be a matter of personal preference, patients intolerant or noncompliant with
one formulation may do better with the other. Fasting lipid profiles and growth
and maturation should be monitored every 6–12 months. No other laboratory
testing for safety is required.
The bile acid-binding sequestrants may be used in
combination with a statin for patients who fail to meet LDL–C target
levels with either medication alone. One pediatric study assessed this
combination and showed no increase in adverse effects.[148] As expected, the efficacy of
the two agents together appears to be additive. Compliance should be closely
monitored, since noncompliance with bile acid sequestrants may cause some
patients to become noncompliant with the more effective statin as well.
Cholesterol Absorption Inhibitors
Ezetimibe, a cholesterol absorption inhibitor, lowers
LDL–C levels by upregulating LDL–C receptors in a manner similar to
the bile acid sequestrants, although the mechanism of action differs.
Intestinal cholesterol absorption is inhibited at the level of the intestinal
villus, resulting in inhibition of absorption of dietary cholesterol.
Absorption of bile salts occurs in the ileum by a different mechanism and is
not affected by the use of cholesterol absorption inhibitors. Studies in adults
have shown that the effect is additive when used with statins, giving an
additional 20 percent lowering of LDL–C levels. Adverse effects are
minimal, and a dose of 10 mg/d is recommended. At present, there is no evidence
that the addition of ezetimibe to a statin provides benefit in the prevention
of atherosclerosis or events, and there are no published studies of its use as
monotherapy in children. A study of ezetimibe for patients with hoFH, including
some children, showed good efficacy and safety regarding lipid lowering.[155] A recent RCT of pediatric
subjects ages 10–17 years with FH demonstrated that coadministered
ezetimibe and simvastatin resulted in significantly greater reductions in
LDL–C levels than did simvastatin alone; the combination was safe and
well-tolerated for up to 53 weeks.[154] Currently, ezetimibe
might be considered for children and adolescents who, under the care of a lipid
specialist, do not meet LDL–C therapeutic targets on a statin alone or as
potential monotherapy for patients with lower LDL–C levels who meet the
criteria for pharmacologic therapy. In 2009, the U.S. Food and Drug
Administration released a safety alert about potential adverse effects of
ezetimibe in combination with statins in adults. Until more data are available
on its safety, ezetimibe should be used only in consultation with a lipid
specialist. Results of future trials should clarify safety issues surrounding
use of ezetimibe in children.
CONCLUSIONS AND GRADING OF THE EVIDENCE REVIEW FOR
USE OF MEDICATION TO TREAT DYSLIPIDEMIA
When medication is recommended, this should
always be in the context of the complete CV risk profile of the patient and in
consultation with the patient and the family.
NOTE: Values given are in mg/dL. To
convert to SI units, divide the results for total cholesterol (TC), low-density
lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C),
and non-HDL-C by 38.6; for triglycerides (TG), divide by 88.6.
- Decisions regarding the need for medication therapy
should be based on the average of results from at least two fasting lipid
profiles obtained at least 2 weeks but no more than 3 months apart (Grade C)
(Figure 9–1).
- The cut points used to define the level at which
drug therapy should be considered from the 1992 NCEP Pediatric
Guidelines have been used as the basis for multiple drug safety and
efficacy trials in dyslipidemic children (Grade B):
- LDL–C ≥ 190 mg/dL after a 6-month
trial of lifestyle management (CHILD 1 à CHILD 2-LDL) for children ages
≥ 10 years.
- LDL–C 160–189 mg/dL after a 6-month
trial of lifestyle/diet management (CHILD 1 à CHILD 2-LDL) in a child
≥ age 10 years with a positive family history of premature CVD/events in
first-degree relatives (Table 9–6) or at least one high-level risk factor
or risk condition or at least 2 moderate-level risk factors or risk conditions
(Tables 9–6, 9–7, and 9–12) (Figure 9–1).
- LDL–C 130–190 mg/dL in a child
≥ age 10 years with a negative family history of premature CVD in
first-degree relatives and no high-level or moderate-level risk factor or risk
condition, management should continue to focus on lifestyle changes (CHILD
1à CHILD 2-LDL) based on lipid profile findings (Figure 9–1) plus
weight management if BMI is at least the 85th percentile.
- The goal of LDL-lowering therapy in childhood
and adolescence is LDL–C below the 95th percentile (≤130
mg/dL).
- Children with homozygous FH and extremely elevated
LDL–C levels (>500 mg/dL) have undergone effective LDL-lowering therapy
with biweekly LDL apheresis under the care of lipid specialists in academic
medical centers (Grade C).
- Multiple cohort studies have shown benefits of
LDL-lowering therapy in children at high risk for accelerated atherosclerosis
(Table 9-7). Children and adolescents with chronic kidney disease, T1DM or
T2DM, Kawasaki disease with coronary aneurysms, or postcardiac transplantation)
should be considered for initiation of medication therapy. (Grade C) (see
Section XI. Diabetes Mellitus and Other Conditions Predisposing to the
Development of Accelerated Atherosclerosis).
- The bile acid sequestrants are medications that
bind bile salts within the intestinal lumen and prevent their enterohepatic
reuptake in the terminal ileum, resulting in a depletion of bile salts in the
liver and a signal for increased production. Since bile salts are synthesized
from intracellular cholesterol in the liver, the intracellular pool of
cholesterol becomes depleted, signaling increased production of LDL receptors
and increased clearance of circulating LDL–C to replenish the
intracellular cholesterol pool for increased production of bile salts. Studies
of bile acid sequestrants in children and adolescents ages 6–18 years with
LDL–C levels from 131 to 190 mg/dL show TC reduction of 7-17 percent and
reduction of LDL–C of 10–20 percent, sometimes with a modest
elevation in TG levels. The bile acid sequestrants commonly have
gastrointestinal side effects, and these significantly affect compliance.
However, they are safe and moderately effective (Grade A).
- Statin medications inhibit hydroxymethylglutaryl
coenzyme A reductase, which is a rate-limiting enzyme in the endogenous
cholesterol synthesis pathway. This results in a decrease in the intracellular
pool of cholesterol, which signals upregulation of LDL receptors and increased
clearance of circulating LDL–C. As a group, statins have been shown
to reduce LDL–C in children and adolescents with marked LDL–C
elevation or FH (defined as elevated LDL–C in the child in
conjunction with a family history of elevated LDL–C and/or atherosclerosis
or CAD) when used from 8 weeks to 2 years for children ages 8–18 years.
The lower LDL–C level for eligibility into the statin trials was
>190 mg/dl or > 160 mg/dl with 2 or more additional risk
factors, after a trial period on diet. Trial subjects were monitored carefully
throughout treatment; adverse impacts on growth, development, or sexual
maturation were not seen, and adverse event profiles and efficacy were similar
to those in studies of adults (Grade A).
- Adverse effects from statins are rare at standard
doses but include myopathy and hepatic enzyme elevation. In the meta-analysis
of statin use in children, evidence of hepatic enzyme elevation and muscle
toxicity did not differ between the statin and placebo groups. Routine
monitoring of hepatic enzymes and clinical assessment for muscle toxicity are
strongly recommended for children and adolescents on statin therapy (Table
9–12). The risk of adverse events increases with use of higher doses and
interacting drugs, the latter occurring primarily with drugs that are
metabolized by the cytochrome P–450 system, which is the primary mode of
metabolism for the majority of statins. Drugs that potentially interact with
statins include fibrates, azol antifungals, macrolide antibiotics,
antiarrhthymics, and protease inhibitors (Grade A).
- Bile acid-binding sequestrants may be used in
combination with a statin for patients who fail to meet LDL–C target
levels with either medication alone. One pediatric study assessed this
combination and showed no increase in adverse effects. The efficacy of the two
agents together appears to be additive (Grade B).
- There is limited published experience in children
with use of niacin and fibrates, which have been useful in treating adult
patients with combined dyslipidemias. Efficacy and safety data are limited, and
no data are available regarding newer formulations. In adults, cholesterol
absorption inhibitors have been advocated as an adjunct to statin therapy for
patients who do not reach LDL–C therapeutic targets. Since their action is
independent of and complementary to that of statins, the LDL–C-lowering
effect is additive. No pediatric studies of monotherapy with cholesterol
absorption inhibitors had been published during the time period for this
evidence review. Use of niacin, fibrates, and cholesterol absorption inhibitors
should be instituted only in consultation with a lipid specialist (Grade
C).
- Medication therapy is rarely needed for children
with elevated TG who respond well to weight loss and lifestyle changes (Grade
B). When TG levels exceed 500 mg/dL, patients are at risk for pancreatitis and
require care in consultation with a lipid specialist (Grade B). In adults, use
of omega-3 fish oil has been shown to lower TG by 30–40 percent and to
raise HDL by 6–17 percent. Experience with fish oil in children is limited
to small case series with no safety concerns identified; there have been no
RCTs of fish oil in children (Grade D).
AGE-BASED RECOMMENDATIONS FOR MEDICATION THERAPY OF
CHILDREN WITH DYSLIPIDEMIA
Children Younger Than Age 10 Years
- Children younger than age 10 years should not be
treated with a medication unless they have a severe primary hyperlipidemia or a
high-risk condition that is associated with serious medical morbidity
(homozygous hypercholesterolemia/ LDL–C ≥400 mg/dL; primary
hypertriglyceridemia with TG ≥500 mg/dL; evident CVD in the first two
decades of life; post cardiac transplantation) (Grade C).
Children Ages 10–21 Years (see algorithms,
Figures 9–1 and 9–2)
- Decisions regarding the need for medication therapy
should be based on the average of results from ≥ two FLPs obtained at
least 2 weeks but no more than 3 months apart. (Grade C) (Figure 9–1)
- Children with average LDL–C ≥ 250 mg/dL
or average TG > 500 mg/dL should be referred directly to a lipid
specialist (Grade B).
- Children with lipid abnormalities should have a
detailed family history taken and be assessed for causes of hyperlipidemia,
additional risk factors, and risk conditions (Grade C) (Tables 9–3,
9–6, and 9–7).
- Children with lipid abnormalities (other than
LDL–C ≥250 mg/dL or TG ≥500 mg/dL) should be initially managed
for 3–6 months with diet changes (CHILD 1àCHILD 2-LDL or CHILD
2-TG, Table 9–8) based on specific lipid profile findings (Figures
9–1 and 9–2); if BMI is ≥ 85th percentile, add increased
physical activity, reduce screen time, and restrict calories. Assessment for
associated secondary causes (Table 9–3), additional risk factors, or
high-risk conditions (Tables 9–6 and 9–7) is recommended. Children at
high risk who are unlikely to achieve lipid targets with this strategy alone
(severe primary dyslipidemia, cardiac transplantation) should concomitantly be
considered for initiation of medication therapy (Grade C) (Section XI. Diabetes
Mellitus and Other Conditions Predisposing to the Development of Accelerated
Atherosclerosis).
LDL–C: Treatment for children with
severe elevation of LDL–C is based on assessment of lipid levels and
associated risk factors or risk conditions (Tables 9–6 and 9–7;
Figures 9–1 and 9–2):
- Children with average LDL–C ≥ 250 mg/dL
should be referred directly to a lipid specialist (Grade B).
- If LDL–C remains ≥ 190 mg/dL after a
6-month trial of lifestyle/diet management (CHILD 1→CHILD 2-LDL) for
children ages 10 years and older, statin therapy should be considered (Grade A)
(Figure 9–1) (Table 9–12).
- If LDL–C remains 130–190 mg/dL in a child
age 10 years or older with a negative family history of premature CVD in
first-degree relatives and no high-level or moderate-level risk factor or risk
condition (Tables 9–6 and 9–7), management should continue to focus
on diet changes (CHILD 2-LDL) based on lipid profile findings (Figure 9–1)
plus weight management if BMI is ≥ 85th percentile. Pharmacologic therapy
is not generally indicated, but treatment with bile acid sequestrants might be
considered, the latter in consultation with a lipid specialist (Grade B).
- If LDL–C remains 160–189 mg/dL after a
trial of lifestyle/diet management (CHILD 1→CHILD 2-LDL) in a child age 10
years or older with a positive family history of premature CVD/events in
first-degree relatives (Table 9–6) or at least one high-level risk factor
or risk condition or at least two moderate-level risk factors or risk
conditions (Tables 9–6 and 9–7), then statin therapy should be
considered (Grade B) (Figure 9–1) (Table 9–12).
- If LDL–C remains ≥ 130 to 159 mg/dL
after a trial of lifestyle/diet management (CHILD 1→CHILD 2-LDL) in a
child age 10 years or older with at least two high-level risk factors or risk
conditions or at least one high-level risk factor or risk condition together
with at least two moderate-level risk factors or risk conditions (Tables
9–6 and 9–7), then statin therapy should be considered (Grade C)
(Figure 9–1) (Table 9–12).
- For children ages 8 and 9 years with LDL–C
persistently ≥ 190 mg/dL after a trial of lifestyle/diet management
(CHILD 1→CHILD 2-LDL), together with multiple first-degree family members
with premature CVD/events, or the presence of at least one high-level risk
factor or risk condition or the presence of at least two moderate-level risk
factors or risk conditions (Figure 9–1) (Tables 9–6 and 9–7),
statin therapy might be considered (Grade B) (Table 9–12).
- Statin use should begin with the lowest available
dose given once daily. If LDL–C target levels are not achieved with at
least 3 months of compliant use, then the dose may be increased by one
increment (usually 10 mg). If LDL–C target levels are still not achieved
with at least 3 months of compliant use, then the dose may be further increased
by one increment. The risk and effectiveness of dose escalation has been
explored in several of the statin clinical trials in children with no
additional safety issues identified (Grade B). Alternatively, a second agent
such as a bile acid sequestrant or cholesterol absorption inhibitor may be
added under the direction of a lipid specialist (Grade B) (Table
9–12).
- Children taking a statin should have routine
clinical monitoring for symptoms of muscle toxicity and assessment of hepatic
transaminases and creatine kinase (Grade A) (Table 9–12).
- Pediatric care providers should be on the alert
for, and children and their families should be counseled about, potential
medication interactions (Grade D) (Table 9–12).
- Females taking a statin should be counseled about
risks associated with pregnancy and appropriate contraception strategies if
indicated. Use of oral contraceptives in combination with statins is not
contraindicated (Grade D) (Table 9–12).
TG, non-HDL–C: Children with
elevated TG or elevated non-HDL–C after control of LDL–C are managed
based on lipid levels (Figure 9–2):
- Children with average fasting levels of TG ≥
500 mg/dL or any single measurement ≥ 1,000 mg/dL related to a primary
hypertriglyceridemia should be treated in conjunction with a lipid specialist;
the CHILD 2-TG diet (Table 9–8) should be started and use of fish oil,
fibrate, or niacin to prevent pancreatitis should be considered (Grade D) (
Figure 9–2) (Tables 9–10 and 9–11).
- Children with fasting levels of TG 200–499
mg/dL after a trial of lifestyle/diet management with CHILD 1→CHILD 2-TG
(Table 9–8) should have non-HDL recalculated and be managed to a goal of
less than 145 mg/dL (Grade D).
- Children with fasting levels of TG 200–499
mg/dL, non-HDL ≥ 145 mg/dL, after a trial of lifestyle/diet management
with CHILD 1→CHILD 2-TG (Table 9–8), including increased fish intake,
may be considered for fish oil supplementation (Grade D) (Table
9–10).
- Children ages 10 years or older with non-HDL–C
levels ≥145 mg/dL after the LDL–C goal is achieved may be considered
for further intensification of statin therapy or additional therapy with a
fibrate or niacin, in conjunction with referral to a lipid specialist (Grade D)
(Figure 9–1) (Tables 9–10 and 9–11).
- Children with severe or complex mixed
dyslipidemias, particularly where multiple medications are being considered,
should be referred for consultation with a lipid specialist (Grade D) (Figures
9–1 and 9–2).
The age-specific recommendations for pharmacologic
management of dyslipidemia are summarized in Table 9–9.
Table 9–9. Evidence-Based Recommendations for
Pharmacologic Treatment of Dyslipidemia
Grades reflect the findings of the
evidence review.
Recommendation levels reflect the
consensus opinion of the Expert Panel.
When medication is
recommended, this should always be in the context of the complete
cardiovascular risk profile of the patient and in consultation with the patient
and the family.
NOTE: Values given are in mg/dL. To
convert to SI units, divide the results for total cholesterol (TC), low-density
lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C),
and non-HDL-C by 38.6; for triglycerides (TG), divide by 88.6.
| Birth–10 years |
Pharmacologic treatment is limited
to children with severe primary hyperlipidemia (homozygous familial
hypercholesterolemia, primary hypertriglyceridemia with TG ≥500 mg/dL) or
a high-risk condition (Tables 9–6 and 9–7) or evident cardiovascular
disease; all under the care of a lipid specialist. |
Grade
C
Recommend |
| ≥10–21 years |
Detailed family history (FHx) and
risk factor (RF) assessment required before initiation of drug therapy.* High- to moderate-level RFs and risk conditions
(RCs) in Tables 9–6 and 9–7. |
Grade
C
Strongly recommend |
| ≥10–21 years (cont.d) |
LDL–C: |
|
| ≥10–21 years (cont.d) |
If average LDL–C ≥250
mg/dL*, consult lipid specialist. |
Grade
B
Strongly recommend |
| ≥10–21 years (cont.d) |
If average LDL–C
≥130–250 mg/dL, or non-HDL ≥145
mg/dL:
- Refer to dietitian for medical nutrition
therapy with Cardiovascular Health Integrated Lifestyle Diet (CHILD 1) →
CHILD 2-LDL (Table 9–8) × 6 months → repeat fasting lipid panel
(FLP)
|
Grade
A
Strongly recommend |
| ≥10–21 years (cont.d) |
Repeat FLP: |
|
| ≥10–21 years (cont.d) |
- → LDL–C <130 mg/dL, continue
CHILD 2- LDL, reevaluate in 12
months
|
Grade
A
Strongly recommend |
| ≥10–21 years (cont.d) |
- → LDL–C ≥190** mg/dL, consider initiation of statin therapy
per Tables 9–11 and
9–12
|
Grade
A
Strongly recommend |
| ≥10–21 years (cont.d) |
- → LDL–C ≥130–189 mg/dL,
FHx (-), no other RF or RC, continue CHILD 2-LDL, reevaluate q. 6 months
|
Grade
B
Recommend |
| ≥10–21 years (cont.d) |
- → LDL–C = 160–189 mg/dL + FHx
positive OR ≥1 high-level RF/RC OR ≥2 moderate-level RFs/RCs,
consider statin therapy per Tables 9–11 and 9–12
|
Grade
B
Recommend |
| ≥10–21 years (cont.d) |
- → LDL–C ≥130–159 mg/dL +
≥2 high-level RFs/RCs OR 1 high-level + 2 moderate-level RFs/RCs,
consider statin therapy per Tables 9–11 and 9–12
|
Grade
B
Recommend |
| ≥10–21 years (cont.d) |
Children on statin therapy
should be counseled and carefully monitored per Table 9–12. |
Grade
A
Strongly recommend |
| ≥10–21 years |
Detailed FHx and RF/RC assessment
required before initiation of drug therapy.*** High- and moderate-level RFs/RCs in Tables
9–6 and 9–7† |
Grade
C
Strongly recommend |
| ≥10–21 years (cont.d) |
TG: |
|
| ≥10–21 years (cont.d) |
If average TG ≥500 mg/dL,
consult lipid specialist |
Grade
B
Recommend |
| ≥10–21 years (cont.d) |
If average TG ≥100 mg/dL in a
child <10 years, ≥130 mg/dL in a child age 10–19 years, <500
mg/dL: |
|
| ≥10–21 years (cont.d) |
- Refer to dietitian for medical nutrition
therapy with CHILD 1 → CHILD 2-TG (Table 9–8) × 6 months
|
Grade
B
Strongly recommend |
| ≥10–21 years (cont.d) |
Repeat fasting lipid
profile: |
|
| ≥10–21 years (cont.d) |
- → TG <100 (130) mg/dL, continue CHILD
2-TG, monitor q. 6–12 months
|
Grade
B
Strongly recommend |
| ≥10–21 years (cont.d) |
- → TG >100 (130) mg/dL, reconsult
dietitian for intensified CHILD 2 TG diet counseling
|
Grade
C
Recommend |
| ≥10–21 years (cont.d) |
- → TG ≥200–499 mg/dL, non-HDL
≥145 mg/dL, consider fish oil +/- consult lipid specialist
|
Grade
D
Recommend |
| ≥10–21 years (cont.d) |
Non-HDL: |
|
| ≥10–21 years (cont.d) |
Children ≥10 years with
non-HDL–C ≥145 mg/dL after LDL–C goal achieved may be
considered for additional treatment with statins, fibrates, or niacin in
conjunction with a lipid specialist. |
Grade
D
Optional |
* Consideration
of drug therapy based on the average of ≥2 FLPs, obtained at least 2
weeks but no more than 3 months apart.
** If average LDL–C ≥190 mg/dL
after CHILD 2-LDL and child is age 8–9 years with + FHx OR ≥1
high-level RF/RC OR ≥ 2 moderate-level RFs/RCs, statin therapy may be
considered.
*** Consideration of drug
therapy based on the average of ≥2 fasting lipid profiles obtained at
least 2 weeks but no more than 3 months apart.
† If child is obese, nutrition therapy
should include calorie restriction and increased activity beyond that
recommended for all children. See Section X. Overweight and Obesity for
additional age-specific recommendations.
Table 9–10. Medications for Managing
Hyperlipidemia
|
Type of Medication |
Mechanism of Action |
Major Effects |
Examples |
Adverse Reactions |
FDA Approval in Youths as of This
Writing |
|
HMG CoA reductase inhibitors (statins) |
Inhibits cholesterol synthesis in hepatic cells,
decreases cholesterol pool, resulting in upregulation of LDL receptors |
Mainly lowers LDL-C; some decrease in TG and
modest increase in HDL-C |
Atorvastatin
Fluvastatin
Lovastatin
Pravastatin
Rosuvastatin
Simvastatin |
Raised hepatic enzymes, raised creatine kinase,
myopathy possibly progressing to rhabdomyolysis |
All statins listed approved as an adjunct to
diet to lower LDL-C in adolescent boys and postmenarchal girls ages 10-18 years
(8+ years for pravastatin) with heFH and LDL-C ≥190 mg/dL, or ≥160
mg/dL with FHx of premature CVD and 2+ CVD risk factors in the pediatric
patient |
|
Bile acid sequestrants |
Binds intestinal bile acids interrupting
enterohepatic recirculation, more cholesterol converted into bile acids,
decreases hepatic cholesterol pool, upregulates LDL receptors |
Lowers LDL-C; small increase in HDL; raises
TG |
Cholestyramine
Colestipol
Colesevelam
|
Limited to gastrointestinal tract: gas, bloating
constipation, cramps |
No pediatric indication listed for
cholestyramine or colestipol; colesevelam indicated as monotherapy or with
statin for LDL-C reduction in boys and postmenarchal girls ages 10-17 years
with FH after diet trial if LDL-C ≥190 mg/dL or if LDL-C ≥160 mg/dL
with FHx premature CVD or 2+ more CVD risk factors in the pediatric
patient |
|
Cholesterol absorption inhibitors |
Inhibits intestinal absorption of cholesterol
and plant sterols, decreases hepatic cholesterol pool, upregulates LDL
receptors |
Mainly lowers LDL-C; some decrease in TG and
small increase in HDL-C |
Ezetimibe |
Myopathy, gastrointestinal upset,
headache |
No |
|
Fibric acid derivatives |
Agonist for PPAR alpha nuclear receptors that
upregulate LPL and downregulate apoC-III, both increasing degradation of VLDL-C
and TG. Hepatic synthesis of VLDL-C may also be decreased. |
Mainly lowers TG and raises HDL-C, with little
effect on LDL-C |
Fenofibrate Gemfibrozil |
Dyspepsia, constipation, myositis,
anemia |
No |
|
Nicotinic acid (extended release) |
Inhibits release of FFA from adipose tissue;
decreases VLDL-C and LDL-C production and HDL-C degradation |
Lowers TG and LDL-C and raises HDL-C; can
decrease Lp(a) |
Niacin, extended release |
Flushing, hepatic toxicity, can increase fasting
blood glucose, uric acid; hyperacidity |
Use not recommended in children < age 2
years |
|
Omega-3 fish oil |
Decreases hepatic FA and TG synthesis while
enhancing FA degradation/oxidation, with subsequent reduced VLDL-C
release |
Lowers TG, raises HDL-C, increases LDL-C and
LDL-C particle size |
Omega-3 acid ethyl esters |
Occasional gastrointestinal side effects but no
adverse effect on glucose levels or muscle or liver enzymes or
bleeding |
Only one FDA-approved fish oil preparation for
adults, but many generic fish oil capsules commercially available |
Table 9–11. Clinical Trials of Lipid-Lowering
Medication Therapy in Children and Adolescents
Bile acid binding resins
|
Study |
Medication |
Subjects/Gender/
Condition |
Daily Dose |
Effect on Lipid
Profile
TC |
Effect on Lipid
Profile
LDL–C |
Effect on Lipid
Profile
HDL–C |
Effect on Lipid Profile
TG |
|
Tonstad et al.
RCT 1 year |
Cholestyramine |
72/both/FH
(LDL ≥ 190
mg/dL without FHx premature CVD or LDL ≥160 with FHx after 1-year diet;
ages 6–11 years) |
8 g |
-12% |
-17% |
+8% |
NA |
|
McCrindle et al
RCT crossover 2 × 8
weeks |
Cholestyramine |
40/both/FH
(1 parent with FH;
LDL–C ≥131 mg/dL; on diet; ages 10–18 years) |
8 g |
-7 to -11% |
-10 to -15% |
+2 to +4% |
+6 to +9% |
|
Tonstad et al
RCT 8 weeks; open-label
44–52 weeks |
Colestipol |
66/both/FH
(TC ≥239
mg/dL and TG ≤115 mg/dL; ages 10–16 years) |
2–12 g |
-17% |
-20% |
-7% |
-13% |
|
McCrindle et al
RCT crossover
2 ×
18 weeks |
Colestipol |
36/both/FH/FCHL
(LDL
≥160 mg/dL after 6 months diet counseling; ages 8–18 years)
|
10 g |
-7% |
-10% |
+2% |
+12% |
|
Stein et al |
Colesevelam |
191/both/ FH
(LDL≥190mg/dL or LDL≥ plus 2 additional RFs after 6 months diet
counseling; ages 10-17 years. |
1.875 g |
-3% |
-6% |
+5% |
+6% |
|
Stein et al (cont.d) |
Colesevelam (cont.d) |
191/both/ FH
(LDL≥190mg/dL or LDL≥ plus 2 additional RFs after 6 months diet
counseling; ages 10-17 years. |
3.75 g |
-7% |
-13% |
+8% |
+5% |
HMG CoA reductase inhibitors (statins)
|
Study |
Medication |
Subjects/Gender/Condition |
Daily Dose |
Effect on Lipid
Profile
TC |
Effect on Lipid
Profile
LDL–C |
Effect on Lipid
Profile
HDL–C |
Effect on Lipid Profile
TG |
|
McCrindle et al.
RCT; open-label 26
weeks |
Atorvastatin |
187/both/FH/
Severe
hyperlipidemia
(LDL–C ≥190 mg/dL or ≥160 mg/dL with FHx;
and TG <400 mg/dL; ages 10–17 years) |
10–20 mg |
-30% |
-40% |
+6% |
-13% |
|
Van der Graaf et al
Open-label
2
years |
Fluvastatin |
85/both/FH
(LDL–C
≥190 mg/dL or LDL–C ≥160 mg/dL and 1+ risk factor or LDL
receptor mutation; ages 10–16 years) |
80 mg |
-27% |
-34% |
+5% |
-5% |
|
Lambert et al.
RCT
8 weeks |
Lovastatin |
69/males/FH
(LDL–C
>95th percentile, FHx atherosclerosis and hyperlipidemia; on diet; mean age
13 years) |
10 mg |
-17% |
-21% |
+9% |
-18% |
|
Lambert et al.
RCT
8 weeks
(cont.d) |
Lovastatin (cont.d) |
69/males/FH
(LDL–C
>95th percentile, FHx atherosclerosis and hyperlipidemia; on diet; mean age
13 years) |
20 mg |
-19% |
-24% |
+2% |
+9% |
|
Lambert et al.
RCT
8 weeks
(cont.d) |
Lovastatin (cont.d) |
69/males/FH
(LDL–C
>95th percentile, FHx atherosclerosis and hyperlipidemia; on diet; mean age
13 years) |
30 mg |
-21% |
-27% |
+11% |
+3% |
|
Lambert et al.
RCT
8 weeks
(cont.d) |
Lovastatin (cont.d) |
69/males/FH
(LDL–C
>95th percentile, FHx atherosclerosis and hyperlipidemia; on diet; mean age
13 years) |
40 mg |
-29% |
-36% |
+3% |
-9% |
|
Stein et al.
RCT
48 weeks |
Lovastatin |
132/males/FH
(LDL
189–503 mg/dL + FHx of high LDL; or 220–503 mg/dL + FHx CAD death;
AHA diet 4+ months; ages 10–17 years) |
10 mg |
-13% |
-17% |
+4% |
+4% |
|
Stein et al.
RCT
48 weeks
(cont.d) |
Lovastatin (cont.d) |
132/males/FH
(LDL
189–503 mg/dL + FHx of high LDL; or 220–503 mg/dL + FHx CAD death;
AHA diet 4+ months; ages 10–17 years) |
20 mg |
-19% |
-24% |
+4% |
+8% |
|
Stein et al.
RCT
48 weeks
(cont.d) |
Lovastatin (cont.d) |
132/males/FH
(LDL
189–503 mg/dL + FHx of high LDL; or 220–503 mg/dL + FHx CAD death;
AHA diet 4+ months; ages 10–17 years) |
40 mg |
-21% |
-27% |
+5% |
+6% |
|
Clauss et al.
RCT
24 weeks |
Lovastatin |
54/females/FH
(FHx FH; LDL
160–400 mg/dL and TG <350 mg/dL; 4-week diet placebo run-in and 20-week
tx; ages 10–17 years, postmenarchal) |
40 mg |
-22% |
-27% |
+3% |
-23% |
|
Knipscheer et al.
RCT
12 weeks |
Pravastatin |
72/ both/FH
(FHx
hypercholesterol or premature atherosclerosis; LDL >95th percentile; diet
× 8 weeks; ages 8–16 years) |
5 mg |
-18% |
-23% |
+4% |
+2% |
|
Knipscheer et al.
RCT
12 weeks
(cont.d) |
Pravastatin (cont.d) |
72/ both/FH
(FHx
hypercholesterol or premature atherosclerosis; LDL >95th percentile; diet
× 8 weeks; ages 8–16 years) |
10 mg |
-17% |
-24% |
+6% |
+7% |
|
Knipscheer et al.
RCT
12 weeks
(cont.d) |
Pravastatin (cont.d) |
72/ both/FH
(FHx
hypercholesterol or premature atherosclerosis; LDL >95th percentile; diet
× 8 weeks; ages 8–16 years) |
20 mg |
-25% |
-33% |
+11% |
+3% |
|
Wiegman et al.
RCT
2 years |
Pravastatin |
214/both/FH
(LDL–C
≥155 mg/dL and TG ≤350 mg/dL; diet × 3 months; ages 8–18
years) |
20–40 mg |
-19% |
-24% |
+6% |
-17% |
|
Rodenburg et al.
Open-label
2-year RCT;
4.5 year open-label followup |
Pravastatin |
186/both/FH
(LDL–C
≥154 mg/dL and TG <154 mg/dL; 3 months on diet; ages 8–18
years) |
20 mg (ages <14 years) or 40
mg (ages >14 years) |
-23% |
-29% |
+3% |
-2% |
|
de Jongh et al.
RCT
48 weeks |
Simvastatin |
173/both/FH
(LDL–C:
158–397 mg/dL; ages 10–17 years) |
10–40 mg |
-31% |
-41% |
+3% |
-9% |
|
de Jongh et al.
RCT
28 weeks |
Simvastatin |
50/both/FH
(LDL–C above
95th percentile, FHx hyperlipidemia, or LDL receptor mutation; ages 9–18
years) |
40 mg |
-30% |
-40% |
+5% |
-17% |
|
Avis et al
RCT 12 weeks; then, 40 week open
label followup |
Rosuvastatin |
177/both/FH
(LDL-C≥190
mg/dL or LDL-C>160 mg/dL plus (+)FHx of early CVD or ≥ 2 other RFs for
CVD |
5 mg |
-30% |
-38% |
+4% |
-13% |
|
Avis et al
RCT 12 weeks; then, 40 week open
label followup (cont.d) |
Rosuvastatin (cont.d) |
177/both/FH
(LDL-C≥190
mg/dL or LDL-C>160 mg/dL plus (+)FHx of early CVD or ≥ 2 other RFs for
CVD |
10 mg |
-34% |
-45% |
+10% |
-15% |
|
Avis et al
RCT 12 weeks; then, 40 week open
label followup (cont.d) |
Rosuvastatin (cont.d) |
177/both/FH
(LDL-C≥190
mg/dL or LDL-C>160 mg/dL plus (+)FHx of early CVD or ≥ 2 other RFs for
CVD |
20 mg |
-39% |
-50% |
+9% |
-16% |
Other agents
|
Study |
Medication |
Subjects/Gender/Condition |
Daily Dose |
Effect on Lipid
Profile
TC |
Effect on Lipid
Profile
LDL–C |
Effect on Lipid
Profile
HDL–C |
Effect on Lipid
Profile
TG |
|
Wheeler et al.
RCT
26 weeks |
Bezafibrate |
14/both/FH
(TC >269 mg/dL,
nl TG + FHx of FH or premature CAD; ages 4–15 years) |
10–20 mg |
-22% |
NC |
+15% |
-23% |
|
Colletti et al.
Open-label
1–19
months |
Niacin |
21/both/FH
(mean LDL = 243
± 45 mg/dL on low-fat diet; mean TG = 87 ± 39 mg/dL; ages
4–14 years) |
500–2,200 mg |
-13% |
-17% |
+4% |
+13% |
|
McCrindle et al.
RCT crossover 2 × 18
weeks |
Pravastatin and Colestipol |
36/both/FH/FCHL
(LDL >160
mg/dL +
FHx of FH or premature CAD; TG >177 mg/dL in 10/36; ages
10–18 years) |
Pravastatin, 10 mg (with
Colestipol, 5g) |
-13% |
-17% |
+4% |
+8% |
|
van der Graaf et al.
RCT
6 and 27 weeks;
open-label to 53 weeks |
Simvastatin and Ezetimibe |
248/both/FH
(LDL >159
mg/dL + genotype-confirmed FH or + parental genotype-confirmed FH or + parental
LDL >210 mg/dL or + tendinous xanthomas or LDL >189 mg/dL + FHx of
hypercholesterolemia; ages 10–17 years) |
Simvastatin 10–40 mg with
Ezetimibe 10 mg |
- 38% |
- 49% |
+7% |
-17% |
|
Addendum:
Goldberg et al.
Omega-3 fatty acid review in
adults; no RCTs in children |
Omega-3 fish oils** (1 gram/capsule) |
- |
1–4 g/d |
NC |
+17–31% |
+6–17% |
-30–40% |
ABBREVIATIONS: AHA = American Heart Association; CAD =
coronary artery disease; d = day; FHx = family history; g = grams; mg =
milligrams; NA = not available; NC = not calculated; TC = total cholesterol; FH
= heterozygous familial hypercholesterolemia; FCHL = familial combined
hyperlipidemia; RCT = randomized controlled trial. tx = treatment
** There is only one
FDA-approved fish oil preparation, but there are many generic forms of fish oil
capsules that are commercially available. The University of Wisconsin maintains
a preventive cardiology patient education Web site
http://www.heartdecision.org. The "fish oil" section includes information about
the content of various preparations. The Web site is updated every 6 months (https://www.heartdecision.org/chdrisk/v_hd/patient_edu_docs/Fish_Oil_11-2007.pdf
Table 9–12. Recommendations for Use of HMG- CoA
reductase Inhibitors (Statins) in Children and Adolescents
Patient Selection
- Use algorithm (Figure 9–1) and risk
factor categories (Tables 9–6 and 9–7) to select statin therapy for
patients.
- Include preferences of patient and family in
decision making.
- In general, do not start treatment with
statins before age 10 years (patients with high-risk family history, high-risk
conditions, or multiple risk factors [Tables 9–6 and 9–7] might be
considered for medication initiation at age 10 years or younger.)
- Precaution/contraindication with potentially
interactive medications (cyclosporine, niacin, fibric acid derivatives,
erythromycin, azole antifungals, nefazodone, many HIV protease inhibitors).
Check for potential interaction with all current medications at
baseline.
- Conduct baseline hepatic panel and creatine
kinase (CK) before initiating treatment.
Initiation and Titration
- Choice of particular statin is a matter of
preference. Clinicians are encouraged to develop familiarity and experience
with one of the statins, including dosage regimen and potential drug-drug
interactions.
- Start with the lowest dose once daily,
usually at bedtime. Atorvastatin and rosuvastatin can be taken in the morning
or evening because of their long half-lives.
- Measure baseline CK, alanine aminotransferase
(ALT), and aspartate aminotransferase (AST).
- Instruct the patient to report all potential
adverse effects, especially muscle cramps, weakness, asthenia, and more diffuse
symptoms suggestive of myopathy.
- Advise female patients about concerns with
pregnancy and the need for appropriate contraception.
- Advise about potential future medication
interactions, especially cyclosporine, niacin, fibric acid derivatives,
erythromycin, azole antifungals, nefazodone, and HIV protease inhibitors.
Check for potential interaction whenever any new medication is
initiated.
- Whenever potential myopathy symptoms
present, stop medication and assess CK; determine relation to recent physical
activity. The threshold for worrisome level of CK is 10 times above the upper
limit of reported normal, considering the impact of physical activity. Monitor
the patient for resolution of myopathy symptoms and any associated increase in
CK. Consideration can be given to restarting the medication once symptoms and
laboratory abnormalities have resolved.
- After 4 weeks, measure fasting lipid profile
(FLP), ALT, and AST and compare with laboratory-specific reported normal
values.
- The threshold for worrisome levels of ALT
or AST is ≥ 3 times the upper limit of reported normal.
- Target levels for LDL-C: Minimal < 130
mg/dL; Ideal < 110 mg/dL.
- If target LDL-C levels are achieved and there
are no potential myopathy symptoms or laboratory abnormalities, continue
therapy and recheck FLP, ALT, and AST in 8 weeks and then 3 months.
- If laboratory abnormalities are noted or
symptoms are reported, temporarily withhold the medication and repeat the blood
work in 2 weeks. When abnormalities resolve, the medication may be restarted
with close monitoring.
- If target LDL-C levels are not achieved,
increase the dose by one increment (usually 10 mg) and repeat the blood work in
4 weeks. If target LDL-C levels are still not achieved, dose may be further
increased by one increment or another agent (bile acid sequestrant or
cholesterol absorption inhibitor) may be added under the direction of a lipid
specialist.
Maintenance Monitoring
- Monitor growth (height, weight, and BMI
relative to normal growth charts), sexual maturation, and development.
- Whenever potential myopathy symptoms present,
stop medication and assess CK.
- Monitor fasting lipoprotein profile, ALT, and
AST every 3-4 months in the first year, every 6 months in the second year and
beyond, and whenever clinically indicated.
- Monitor and encourage compliance with
lipid-lowering dietary and medication therapy. Serially assess and counsel for
other risk factors, such as weight gain, smoking, and inactivity.
- Counsel adolescent females about statin
contraindications in pregnancy and the need for abstinence or use of
appropriate contraceptive measures. Use of oral contraceptives is not
contraindicated if medically appropriate. Seek referral to an adolescent
medicine or gynecologic specialist as appropriate.
|
Figure 9-1. Dyslipidemia Algorithm: TARGET LDL-C
(Low-Density Lipoprotein Cholesterol)
NOTE: Values given are in mg/dL. To
convert to SI units, divide results for total cholesterol (TC), low-density
lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C),
and non-HDL-C by 38.6; for triglycerides (TG), divide by 88.6.

Figure 9-1 Description
The figure is a flow chart with 13 labeled boxes
linked by arrows. The chart flows in one direction with arrows pointing
downward and lateral arrows to one or more boxes.
Below the flow chart is described as lists in which
the possible next steps are listed beneath each box label.
- Fasting lipid profile (FLP) x 2*, average
- Forward to LDL-C > 130, < 250 mg/dL
** → Target LDL-C TG > 100,
< 500 mg/dL, < 10 y → Target TG > 130, < 500 mg/dL, 10-19
y
- TG ≥ 500 mg/dL,
→ Consult lipid
specialist
- Lateral to LDL-C ≥ 250 mg/dL →
Consult lipid specialist
- LDL-C ≥ 250 mg/dL
→ Consult lipid
specialist
- Lateral to TG ≥ 500 mg/dL, → Consult
lipid specialist
- LDL-C ≥ 130, < 250 mg/dL
** → Target LDL-C
TG≥
100, < 500 mg/dL, < 10 y → Target TG
≥ 130, < 500
mg/dL, 10-19 y (see TG algorithm, Figure 9-2)
- Forward to FLP
- Boxed text with no arrow between the two boxes:
Exclude secondary causes. Evaluate for other risk factors (RFs). Start
Cardiovascular Health Integrated Lifestyle Diet (CHILD 1) → CHILD
2-LDL (Table 9-8) + lifestyle change x 6 months***
- FLP
- Forward to LDL-C < 130
mg/dL
- Forward to LDL-C ≥130 to
-189 mg/dL Family history (FHx) (-) No other RFs
- Forward to LDL-C ≥ 190
mg/dL
- Forward to LDL-C ≥ 160
to -189 mg/dL FHx (+) or 1 high-level RF or ≥ 2 moderate-level RFs
- Forward to LDL-C ≥ 130
to -159 mg/dL + 2 high-level RFs or 1 high-level + ≥ 2 moderate-level RFs
OR clinical CVD
- LDL-C < 130 mg/dL
→
Continue CHILD 2-LDL
→ Repeat FLP q. 12 months
- LDL-C ≥130 to -189 mg/dL
Family history (FHx) (-) No other RFs
→ Continue CHILD 2 LDL, Follow
q. 6 m with FLP, FHx/ RF update
- LDL-C ≥ 190 mg/dL
→
Initiate statin therapy (Tables 9-11, 9-12)
- Forward to Follow with FLPs, related
chemistries per Table 9-12
- LDL-C ≥ 160 to -189 mg/dL
FHx (+) or 1 high-level RF or ≥ 2 moderate-level RFs
→Initiate
statin therapy (Tables 9-11, 9-12)
- Forward to Follow with FLPs, related
chemistries per Table 9-12
- LDL-C ≥ 130 to -159 mg/dL +
2 high-level RFs or 1 high-level + ≥ 2 moderate-level RFs OR clinical CVD
→ Initiate statin therapy (Tables 9-11, 9-12)
- Forward to Follow with FLPs, related
chemistries per Table 9-12
- Follow with FLPs, related chemistries per Table
9-12
- Forward to → LDL-C still≥130 mg/dL,
TG <200 mg/dL, refer to lipid specialist for addition of second
lipid-lowering agent; monitor per Table 9-12 → In high LDL-C patients, if
non-HDL-C ≥145 mg/dL after effective LDL-C treatment, → Target TG
(Figure 9-2)
- → LDL-C still ≥130 mg/dL, TG <200
mg/dL, refer to lipid specialist for addition of second lipid-lowering agent;
monitor per Table 9-12
→ In high LDL-C patients, if non-HDL-C
≥145 mg/dL after effective LDL-C treatment,
→ Target TG
(Figure 9-2)
Figure 9-1 Footnotes:
* Obtain FLPs
at least 2 weeks but no more than 3 months apart.
** Per Table 5, use of drug therapy is
limited to children ≥10 y with defined risk profiles.
*** In a child with LDL-C > 190 mg/dL
and other RFs, trial of CHILD 2 LDL diet may be abbreviated.
Figure 9-2. Dyslipidemia Algorithm: TARGET TG
(Triglycerides)
NOTE: Values given are in mg/dL. To
convert to SI units, divide results for total cholesterol (TC), low-density
lipoprotein cholesterol (LDL-C) high-density lipoprotein cholesterol (HDL-C),
and non-HDL-C by 38.6; for triglycerides (TG), divide by 88.6.
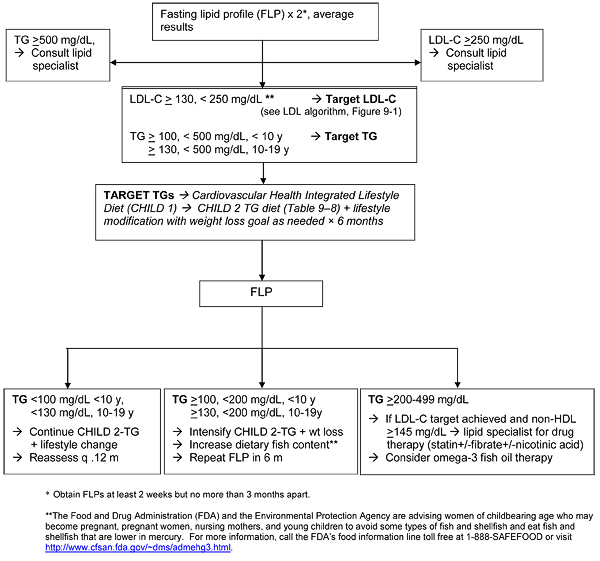
Figure 9-2 Description
The figure is a flow chart with 9 labeled boxes linked
by arrows. The chart flows in one direction with arrows pointing downward and
lateral arrows to one or more boxes.
Below the flow chart is described as lists in which
the possible next steps are listed beneath each box label.
- Fasting lipid profile (FLP) x 2*, average results
- Forward to LDL-C ≥ 130, < 250 mg/dL
** → Target
LDL-C TG≥ 100, < 500 mg/dL, < 10 y → Target
TG ≥ 130, < 500 mg/dL, 10-19 y
- TG ≥ 500 mg/dL,
→ Consult lipid
specialist
- Lateral to LDL-C ≥ 250 mg/dL →
Consult lipid specialist
- LDL-C ≥ 250 mg/dL
→ Consult lipid
specialist
- Lateral to TG ≥ 500 mg/dL, → Consult
lipid specialist
- LDL-C ≥ 130, < 250 mg/dL
** → Target
LDL-C (see LDL algorithm, Figure 9-1)
TG≥ 100, < 500
mg/dL, < 10 y → Target TG
≥ 130, < 500
mg/dL, 10-19 y
- Forward to TARGET TGs →
Cardiovascular Health Integrated Lifestyle Diet (CHILD 1) → CHILD 2 TG
diet (Table 9-8) + lifestyle modification with weight loss goal as needed
× 6 months
- TARGET TGs → Cardiovascular
Health Integrated Lifestyle Diet (CHILD 1) → CHILD 2 TG diet (Table 9-8) +
lifestyle modification with weight loss goal as needed × 6 months
- Forward to FLP
- FLP
- Forward to TG <100 mg/dL
<10 y, <130 mg/dL, 10-19 y
- Forward toTG ≥100,
<200 mg/dL, <10 y ≥130, <200 mg/dL, 10-19y
- Forward to TG ≥200-499
mg/dL
- TG <100 mg/dL <10 y,
<130 mg/dL, 10-19 y
→ Continue CHILD 2-TG + lifestyle change
→Reassess q .12 m
- TG ≥100, <200 mg/dL,
<10 y
≥130, <200 mg/dL, 10-19y
→ Intensify CHILD 2-TG
+ wt loss ' Increase dietary fish content**
→ Repeat FLP in 6 m
- TG ≥200-499 mg/dL
→
If LDL-C target achieved and non-HDL ≥145 mg/dL → lipid specialist
for drug therapy (statin+/-fibrate+/-nicotinic acid)
→ Consider
omega-3 fish oil therapy
Figure 9-2 Footnotes:
* Obtain FLPs
at least 2 weeks but no more than 3 months apart.
** The Food and Drug Administration (FDA)
and the Environmental Protection Agency are advising women of childbearing age
who may become pregnant, pregnant women, nursing mothers, and young children to
avoid some types of fish and shellfish and eat fish and shellfish that are
lower in mercury. For more information, call the FDA's food information line
toll free at 1-888-SAFEFOOD or visit
http://www.cfsan.fda.gov/~dms/admehg3.html.
REFERENCES
[1] NCEP Expert Panel of Blood
Cholesterol Levels in Children and Adolescents. National Cholesterol Education
Program (NCEP): Highlights of the Report of the Expert Panel on Blood
Cholesterol Levels in Children and Adolescents. Pediatrics 1992;89:495-501.
(PM:1741227)
[2] Berenson GS, Srinivasan SR, Bao W,
Newman WP, III, Tracy RE, Wattigney WA. Association between multiple
cardiovascular risk factors and atherosclerosis in children and young adults.
The Bogalusa Heart Study. N Engl J Med 1998;338(23):1650-1656.
(PM:9614255)
[3] McGill HC Jr, McMahan CA, Zieske AW,
Sloop GD, Walcott JV, Troxclair DA, Malcom GT, Tracy RE, Oalmann MC, Strong JP.
Associations of coronary heart disease risk factors with the intermediate
lesion of atherosclerosis in youth. The Pathobiological Determinants of
Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb Vasc Biol
2000;20(8):1998-2004. (PM:10938023)
[4] McGill HC Jr, McMahan CA, Herderick
EE, Tracy RE, Malcom GT, Zieske AW, Strong JP. Effects of coronary heart
disease risk factors on atherosclerosis of selected regions of the aorta and
right coronary artery. PDAY Research Group. Pathobiological Determinants of
Atherosclerosis in Youth. Arterioscler Thromb Vasc Biol 2000;20(3):836-845.
(PM:10712411)
[5] Strong JP, Malcom GT, McMahan CA,
Tracy RE, Newman WP, III, Herderick EE, Cornhill JF. Prevalence and extent of
atherosclerosis in adolescents and young adults: implications for prevention
from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA
1999;281(8):727-735. (PM:10052443)
[6] McMahan CA, Gidding SS, Malcom GT,
Tracy RE, Strong JP, McGill HC, Jr. Pathobiological determinants of
atherosclerosis in youth risk scores are associated with early and advanced
atherosclerosis. Pediatrics 2006;118(4):1447-1455.
(PM:17015535)
[7] McMahan CA, Gidding SS, Fayad ZA,
Zieske AW, Malcom GT, Tracy RE, Strong JP, McGill HC Jr. Risk scores predict
atherosclerotic lesions in young people. Arch Intern Med. 2005;165(8):883-890.
(PM:15851639)
[8] Frontini MG, Srinivasan SR, Xu J,
Tang R, Bond MG, Berenson GS. Usefulness of childhood non-high density
lipoprotein cholesterol levels versus other lipoprotein measures in predicting
adult subclinical atherosclerosis: the Bogalusa Heart Study. Pediatrics.
2008;121(5):924-929. (PM:18450895)
[9] Juonala M, Viikari JS, Rönnemaa
T, Marniemi J, Jula A, Loo BM, Raitakari OT. Associations of dyslipidemias from
childhood to adulthood with carotid intima-media thickness, elasticity, and
brachial flow-mediated dilatation in adulthood: the Cardiovascular Risk in
Young Finns Study. Arterioscler Thromb Vasc Biol 2008;28(5):1012-1017.
(PM:18309111)
[10] Kieltyka L, Urbina EM, Tang R, Bond
MG, Srinivasan SR, Berenson GS. Framingham risk score is related to carotid
artery intima-media thickness in both white and black young adults: the
Bogalusa Heart Study. Atherosclerosis 2003;170(1):125-130.
(PM:12957690)
[11] Mahoney LT, Burns TL, Stanford W,
Thompson BH, Witt JD, Rost CA, Lauer RM. Coronary risk factors measured in
childhood and young adult life are associated with coronary artery
calcification in young adults: the Muscatine Study. J Am Coll Cardiol
1996;27(2):277-284. (PM:8557894)
[12] Li S, Chen W, Srinivasan SR, Bond
MG, Tang R, Urbina EM, Berenson GS. Childhood cardiovascular risk factors and
carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA
2003;290(17):2271-2276. (PM:14600185)
[13] Raitakari OT, Juonala M,
Kähönen M, Taittonen L, Laitinen T, Mäki-Torkko N,
Järvisalo MJ, Uhari M, Jokinen E, Rönnemaa T, Akerblom HK, Viikari
JS. Cardiovascular risk factors in childhood and carotid artery intima-media
thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA
2003;290(17):2277-2283. (PM:14600186)
[14] Juonala M, Viikari JS, Kahonen M,
Solakvi T, Helenius H, Jula A, Marniemi J, Taittonen L, Laitinen T, Nikkari T,
Raitakari OT. Childhood levels of serum apolipoproteins B and A-I predict
carotid intima-media. Thickness and brachial endothelial function in adulthood:
the cardiovascular risk in young Finns study. J Am Coll Cardiol
2008;52(4):293-299. (PM: 18634985)
[15]Frontini MG, Srinivasan SR, Su JH,
Tang R, Bond MG, Berenson G. Utility of non-high-density Lipoprotein
cholesterol versus other lipoprotein measures in detecting subclinical
atherosclerosis in Young adults (The Bogalusa Heart Study). Am J Cardiol
2007;100(1):64-68. (PM:17599442)
[16] Kwiterovich PO, Jr. Recognition and
Management of dyslipidemia in children and adolescents. J Clin Endocrinol Metab
2008;93(11):4200-4209.
[17] Morrison JA, Friedman LA,
Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular
disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study.
Pediatrics 2007;120(2):340-345. (PM:17671060)
[18] Morrison JA, Friedman LA, Wang P,
Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome
and type 2 diabetes mellitus 25 to 30 years later. J Pediatr
2008;152(2):201-206. (PM:18206689)
[19] Thavendiranathan P, Bagai A,
Brookhart MA, Choudhry NK. Primary prevention of cardiovascular diseases with
statin therapy: a meta-analysis of randomized controlled trials. Arch Intern
Med 2006;166(21):2307-2313.
[20] Raitakari OT, Ronnemaa T, Jarvisalo
MJ, Kaitosaari T, Volanen I, Kallio K, Lagstrom H, Jokinen E, Niinikoski H,
Viikari JSA, Simell O. Endothelial function in healthy 11-year-old children
after dietary intervention with onset in infancy. The Special Turku Coronary
Risk factor Intervention Project for Children (STRIP). Circulation
2005;112:3786-3794. (PM:16330680)
[21] Wiegman A, Hutten BA, de Groot E,
Rodenburg J, Bakker HD, Büller HR, Sijbrands EJ, Kastelein JJ. Efficacy
and safety of statin therapy in children with familial hypercholesterolemia: a
randomized controlled trial. JAMA 2004;292(3):331-337.
(PM:15265847)
[22] Rodenburg J, Vissers MN, Wiegman A,
Trotsenburg ASP, van der Graaf A, de Groot E, Wijburg FA, Kastelein JJP, Hutten
BA. Statin treatment in children with familial hypercholesterolemia: the
younger the better. Circulation 2007;116:664-668.
[23] de Jongh S, Lilien MR, op't RJ,
Stroes ES, Bakker HD, Kastelein JJ. Early statin therapy restores endothelial
function in children with familial hypercholesterolemia. J Am Coll Cardiol
2002;40(12):2117-2121. (PM:12505222)
[24] Mudd JO, Borlaug BA, Johnston PV,
Kral BG, Rouf R, Blumenthal RS, Kwiterovich PO Jr. Beyond low-density
lipoprotein cholesterol: defining the role of low-density lipoprotein
heterogeneity in coronary artery disease. J Am Coll Cardiol
2007;50(18):1735-1741.
[25] Bachorik PS, Lovejoy KL, Carroll MD,
Johnson CL. Apolipoprotein B and AI distributions in the United States,
1988-1991: results of the National Health and Nutrition Examination Survey III
(NHANES III). Clin Chem 1997;43(12):2364-2378.
(PM:9439456)
[26] Bao W, Srinivasan SR, Berenson GS.
Tracking of serum apolipoproteins A-I and B in children and young adults: the
Bogalusa Heart Study. J Clin Epidemiol 1993;46(7):609-616.
(PM:8326345)
[27] Sniderman A, Teng B, Genest J,
Cianflone K, Wacholder S, Kwiterovich P, Jr. Familial aggregation and early
expression of hyperapobetalipoproteinemia. Am J Cardiol 1985;55(4):291-295.
[28] Freedman DS, Srinivasan SR, Shear
CL, Franklin FA, Webber LS, Berenson GS. The relation of apolipoproteins A-I
and B in children to parental myocardial infarction. N Engl J Med
1986;315(12):721-726.
[29] ter Avest E, Sniderman AD, Bredie
SJ, Wiegman A, Stalenhoef AF, de Graffe J. Effect of aging and obesity on the
expression of dyslipidaemia in children from families with familial combined
hyperlipidaemia. Clin Sci (Lond) 2007;112(2):131-139.
[30] Srinivasan SR, Myers L, Berenson GS.
Distribution and correlates of non-high-density lipoprotein cholesterol in
children: the Bogalusa Heart Study. Pediatrics 2002;110(3):e29.
(PM:12205279)
[31] Cui Y, Blumenthal RS, Flaws JA,
Whiteman MK, Langenberg P, Bachorik PS, Bush TL. Non-High-Density Lipoprotein
Cholesterol level as a predictor of cardiovascular disease mortality. Arch
Intern Med 2001; 161: 1413-1419.
[32] Srinivasan SR, Frontini MG, Xu J,
Berenson GS. Utility of childhood non-high-density lipoprotein cholesterol
levels in predicting adult dyslipidemia and other cardiovascular risks: the
Bogalusa Heart Study. Pediatrics 2006;118(1):201-206.
(PM:16818566)
[33] Rainwater DL, McMahan CA, Malcom GT,
Scheer WD, Roheim PS, McGill HC Jr, Strong JP. Lipid and apolipoprotein
predictors of atherosclerosis in youth: apolipoprotein concentrations do not
materially improve prediction of arterial lesions in PDAY subjects. The PDAY
Research Group. Arterioscler Thromb Vasc Biol 1999;19(3):753-761.
(PM:10073983)
[34] Srinivasan SR, Ehnholm C, Wattigney
WA, Berenson GS. Influence of apolipoprotein E polymorphism on the tracking of
childhood levels of serum lipids and apolipoproteins over a 6-year period. The
Bogalusa Heart Study. Atherosclerosis 1996;127(1):73-79.
(PM:9006807)
[35] Srinivasan SR, Ehnholm C, Wattigney
WA, Bao W, Berenson GS. The relation of apolipoprotein E polymorphism to
multiple cardiovascular risk in children: the Bogalusa Heart Study.
Atherosclerosis 1996;123(1-2):33-42. (PM:8782835)
[36] Rask-Nissilä L, Jokinen E,
Viikari J, Tammi A, Rönnemaa T, Marniemi J, Salo P, Routi T, Helenius H,
Välimäki I, Simell O. Impact of dietary intervention, sex, and
apolipoprotein E phenotype on tracking of serum lipids and apolipoproteins in
1- to 5-year-old children: the Special Turku Coronary Risk Factor Intervention
Project for Children (STRIP). Arteroscler Thromb Vasc Biol 2002;22(3):492-498.
(PM:11884296)
[37] Tammi A, Rönnemaa T, Miettinen
TA, Gylling H, Rask-Nissilä L, Viikari J, Tuominen J, Marniemi J, Simell
O. Effects of gender, apolipoprotein E phenotype and cholesterol-lowering by
plant stanol esters in children: the STRIP study. Special Turku Coronary Risk
Factor Intervention Project. Acta Paediatr 2002;91(11):1155-1162.
(PM:12463311)
[38] Marcovina SM, Koschinsky ML, Albers
JJ, Skarlatos S. Report of the National Heart, Lung, and Blood Institute
Workshop on Lipoprotein(a) and Cardiovascular Disease: recent advances and
future directions. Clin Chem 2003;49(11):1785-1796.
[39] Jones GT, van Rij AM, Cole J,
Williams MJ, Bateman EH, Marcovina SM, Deng M, McCormick SP. Plasma
lipoprotein(a) indicates risk for 4 distinct forms of vascular disease. Clin
Chem 2007;53(4):679-685.
[40] Srinivasan SR, Dahlen GH, Jarpa RA,
Webber LS, Berenson GS. Racial (black-white) differences in serum lipoprotein
(a) distribution and its relation to parental myocardial infarction in
children. Bogalusa Heart Study. Circulation 1991;84(1):160-167.
(PM:1829398)
[41]Nowak-Gottl U, Langer C, bergs S,
Thedieck S, Strater R, Stoll M. Genetics of hemostasis: differential effects of
hereditability and household components influencing lipid concentrations and
clotting factor levels in 282 pediatric stroke families. Environmental Health
Perspectives 2008; 116:839-843.
[42] Simma B, Martin G, Muller T, Huemer
M. Risk factors for pediatric stroke: consequences for therapy and quality of
life. Pediatr Neurol 2007;37(2):121-126.
[43] Freedman DS, Bowman BA, Otvos JD,
Srinivasan SR, Berenson GS. Levels and correlates of LDL and VLDL particle
sizes among children: the Bogalusa heart study. Atherosclerosis
2000;152(2):441-449.
[44] Cali AM, Zern TL, Taksali SE, de
Oliveira AM, Dufour S, Otvos JD, Caprio S. Intrahepatic fat accumulation and
alterations in lipoprotein composition in obese adolescents: a perfect
proatherogenic state. Diabetes Care 2007; 30:3093-3098.
[45] Freedman DS, Bowman BA, Otvos JD,
Srinivasan SR, Berenson GS. Differences in the relation of obesity to serum
triacylglycerol and VLDL subclass concentrations between black and white
children: the Bogalusa Heart Study. Am J Clin Nutr 2002;75(5):827-833.
(PM:11976155)
[46] Tzou WS, Douglas PS, Srinivasan SR,
Chen W, Berenson G, Stein JH. Advanced lipoprotein testing does not improve
identification of subclinical atherosclerosis in young adults: the Bogalusa
Heart Study. Ann Intern Med 2005;142(9):742-750.
[47] LaRosa JC, Chambless LE, Criqi MH,
Frantz ID, Glueck CJ, Heiss G, Morrison JA. Patterns of dyslipoproteinemia in
selected North American populations: The Lipid Research Clinics program
Prevalence Study. Circulation 1986;73:I12-29.
[48] Hickman TB, Briefel RR, Carroll MD,
Rifkind BM, Cleeman JI, Maurer KR, Johnson CL. Distributions and trends of
serum lipid levels among United States children and adolescents ages 4-19
years: data from the Third National Health and Nutrition Examination Survey.
Prev Med 1998;27(6):879-890. (PM:9922071)
[49] Freedman DS, Srinivasan SR, Cresanta
JL, Webber LS, Berenson GS. Cardiovascular risk factors from birth to 7 years
of age: the Bogalusa Heart Study. Serum lipids and lipoproteins. Pediatrics
1987;80(5 Pt 2):789-796. (PM:3478647)
[50] Kwiterovich PO, Jr., Levy RI,
Fredrickson DS. Neonatal diagnosis of familial type-II hyperlipoproteinaemia.
Lancet 1973;1(7795):118-121.
[51] Webber LS, Srinivasan SR, Wattigney
WA, Berenson GS. Tracking of serum lipids and lipoproteins from childhood to
adulthood. The Bogalusa Heart Study. Am J Epidemiol 1991;133(9):884-899.
(PM:2028978)
[52] Srinivasan SR, Wattigney W, Webber
LS, Berenson GS. Race and gender differences in serum lipoproteins of children,
adolescents, and young adults--emergence of an adverse lipoprotein pattern in
white males: the Bogalusa Heart Study. Prev Med 1991;20(6):671-684.
(PM:1766940)
[53] Magnussen CG, Raitakari OT, Thomson
R, Juonala M, Patel DA, Viikari JS, Marniemi J, Srinivasan SR, Berenson GS,
Dwyer T, Venn A. Utility of currently recommended pediatric dyslipidemia
classifications in predicting dyslipidemia in adulthood: evidence from the
Childhood Determinants of Adult Health (CDAH) study, Cardiovascular Risk in
Young Finns Study, and Bogalusa Heart Study. Circulation 2008;117(1):32-42.
(PM:18071074)
[54] Jolliffe CJ, Janssen I. Distribution
of lipoproteins by age and gender in adolescents. Circulation
2006;114(10):1056-1062. (PM:16940191)
[55] Expert Panel on Detection,
Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive
Summary of the Third Report of the National Cholesterol Education Program
(NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood
Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285(19):2486-2497.
(PM:11368702)
[56] Bao W, Srinivasan SR, Wattigney WA,
Bao W, Berenson GS. Usefulness of childhood low-density lipoprotein cholesterol
level in predicting adult dyslipidemia and other cardiovascular risks. The
Bogalusa Heart Study. Arch Intern Med 1996;156(12):1315-1320.
(PM:8651840)
[57] Jiang X, Srinivasan SR, Webber LS,
Wattigney WA, Berenson GS. Association of fasting insulin level with serum
lipid and lipoprotein levels in children, adolescents, and young adults: the
Bogalusa Heart Study. Arch Intern Med 1995;155(2):190-196.
(PM:7811129)
[58] Stuhldreher WL, Orchard TJ, Donahue
RP, Kuller LH, Gloninger MF, Drash AL. Cholesterol screening in childhood:
sixteen-year Beaver County Lipid Study experience. J Pediatr
1991;119(4):551-556. (PM:1919885)
[59] Porkka KV, Viikari JS, Taimela S,
Dahl M, Akerblom HK. Tracking and predictiveness of serum lipid and lipoprotein
measurements in childhood: a 12-year follow-up. The Cardiovascular Risk in
Young Finns study. Am J Epidemiol 1994;140(12):1096-1110.
(PM:7998592)
[60] Friedman LA, Morrison JA, Daniels
SR, McCarthy WF, Sprecher DL. Sensitivity and specificity of pediatric lipid
determinations for adult lipid status: findings from the Princeton Lipid
Research Clinics Prevalence Program Follow-up Study. Pediatrics
2006;118(1):165-172. (PM:16818562)
[61] Lauer RM, Clarke WR. Use of
cholesterol measurements in childhood for the prediction of adult
hypercholesterolemia. The Muscatine Study. JAMA 1990;264(23):3034-3038.
(PM:2243431)
[62] Haney EM, Huffman LH, Bougatsos C,
Freeman M, Steiner RD, Nelson HD. Screening and treatment for lipid disorders
in children and adolescents: systematic evidence review for the US Preventive
Services Task Force. Pediatrics 2007;120(1):e189-e214.
(PM:17606543)
[63] Gillman MW. Screening for familial
hypercholesterolemia in childhood. Am J Dis Child 147:393-396
[64] Smoak CG, Burke GL, Webber LS,
Harsha DW, Srinivasan SR, Berenson GS. Relation of obesity to clustering of
cardiovascular disease risk factors in children and young adults. The Bogalusa
Heart Study. Am J Epidemiol 1987;125(3):364-372.
(PM:3544817)
[65] Gidding SS, Bao W, Srinivasan SR,
Berenson GS. Effects of secular trends in obesity on coronary risk factors in
children: the Bogalusa Heart Study. J Pediatr 1995;127(6):868-874.
[66] Kikuchi DA, Srinivasan SR, Harsha
DW, Webber LS, Sellers TA, Berenson GS. Relation of serum lipoprotein lipids
and apolipoproteins to obesity in children: the Bogalusa Heart Study. Prev Med
1992;21(2):177-190. (PM:1579553)
[67] Wattigney WA, Harsha DW, Srinivasan
SR, Webber LS, Berenson GS. Increasing impact of obesity on serum lipids and
lipoproteins in young adults. The Bogalusa Heart Study. Arch Intern Med
1991;151(10):2017-2022. (PM:1929690)
[68] Freedman DS, Mei Z, Srinivasan SR,
Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among
overweight children and adolescents: the Bogalusa Heart Study. J Pediatr
2007;150(1):12-17 (PM:17188605)
[69] Sinaiko AR, Donahue RP, Jacobs DR,
Jr., Prineas RJ. Relation of weight and rate of increase in weight during
childhood and adolescence to body size, blood pressure, fasting insulin, and
lipids in young adults. The Minneapolis Children's Blood Pressure Study.
Circulation 1999;99(11):1471-1476. (PM:10086972)
[70] Austin MA, Hokanson JE, Edwards KL.
Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol
1998;81(4A):7B-12B.
[71] Sanders TA. Dietary fat and
postprandial lipids. Curr Atheroscler Rep 2003;5(6):445-451.
[72] Ford ES, Giles WH, Dietz WH.
Prevalence of the metabolic syndrome among US adults: findings from the third
National Health and Nutrition Examination Survey. JAMA 2002;287(3):356-359
[73] Cortner JA, Coates PM, Gallagher PR.
Prevalence and expression of familial combined hyperlipidemia in childhood. J
Pediatr 1990;116(4):514-519.
[74] Boyd GS, Koenigsberg J, Falkner B,
Gidding S, Hassink S. Effect of obesity and high blood pressure on plasma lipid
levels in children and adolescents. Pediatrics 2005;116(2):442-446.
[75] Koenigsberg J, Boyd GS, Gidding SS,
Hassink SG, Falkner B. Association of age and sex with cardiovascular risk
factors and insulin sensitivity in overweight children and adolescents. J
Cardiometab Syndr 2006;1(4):253-258.
[76] Norman JE, Bild D, Lewis CE, Liu K,
West DS. The impact of weight change on cardiovascular disease risk factors in
young black and white adults: the CARDIA study. Int J Obes Relat Metab Disord
2003;27(3):369-376. (PM:12629565)
[77] Gidding SS. Dyslipidemia in the
metabolic syndrome in children. J Cardiometab Syndr 2006;1(4):282-285.
[78] Youssef AA, Srinivasan SR,
Elkasabany A, Chen W, Berenson GS. Trends of lipoprotein variables from
childhood to adulthood in offspring of parents with coronary heart disease: the
Bogalusa Heart Study. Metabolism 2001;50(12):1441-1446.
(PM:11735090)
[79] Chen W, Srinivasan SR, Bao W,
Berenson GS. The magnitude of familial associations of cardiovascular risk
factor variables between parents and offspring are influenced by age: the
Bogalusa Heart Study. Ann Epidemiol 2001;11(8):522-528.
(PM:11709270)
[80] Bao W, Srinivasan SR, Valdez R,
Greenlund KJ, Wattigney WA, Berenson GS. Longitudinal changes in cardiovascular
risk from childhood to young adulthood in offspring of parents with coronary
artery disease: the Bogalusa Heart Study. JAMA 1997;278(21):1749-1
(PM:9388151)
[81] Chen W, Srinivasan SR, Bao W,
Wattigney WA, Berenson GS. Sibling aggregation of low- and high-density
lipoprotein cholesterol and apolipoproteins B and A-I levels in black and white
children: the Bogalusa Heart Study. Ethn Dis 1997;7(3):241-249.
(PM:9467707)
[82] Folsom AR, Burke GL, Ballew C,
Jacobs DR Jr, Haskell WL, Donahue RP, Liu KA, Hilner JE. Relation of body
fatness and its distribution to cardiovascular risk factors in young blacks and
whites. The role of insulin. Am J Epidemiol 1989;130(5):911-924.
(PM:2683750)
[83] Burns TL, Moll PP, Lauer RM.
Increased familial cardiovascular mortality in obese schoolchildren: the
Muscatine Ponderosity Family Study. Pediatrics. 1992;89(2):262-8.
(PM:1734394)
[84] Baumgartner RN, Roche AF, Chumlea
WC, Siervogel RM, Glueck CJ. Fatness and fat patterns: associations with plasma
lipids and blood pressures in adults, 18 to 57 years of age. Am J Epidemiol
1987;126(4):614-628. (PM:3498363)
[85] Freedman DS, Srinivasan SR, Harsha
DW, Webber LS, Berenson GS. Relation of body fat patterning to lipid and
lipoprotein concentrations in children and adolescents: the Bogalusa Heart
Study. Am J Clin Nutr 1989;50(5):930-939. (PM:2816800)
[86] Kavey RE, Allada V, Daniels SR,
Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J; American Heart
Association Expert Panel on Population and Prevention Science; American Heart
Association Council on Cardiovascular Disease in the Young; American Heart
Association Council on Epidemiology and Prevention; American Heart Association
Council on Nutrition, Physical Activity and Metabolism; American Heart
Association Council on High Blood Pressure Research; American Heart Association
Council on Cardiovascular Nursing; American Heart Association Council on the
Kidney in Heart Disease; Interdisciplinary Working Group on Quality of Care and
Outcomes Research. Cardiovascular risk reduction in high-risk pediatric
patients: a scientific statement from the American Heart Association Expert
Panel on Population and Prevention Science; the Councils on Cardiovascular
Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity
and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the
Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of
Care and Outcomes Research: endorsed by the American Academy of Pediatrics.
Circulation 2006;114(24):2710-38. (PM:17130340)
[87] Owen CG, Whincup PH, Odoki K, Gilg
JA, Cook DG. Infant feeding and blood cholesterol: a study in adolescents and a
systematic review. Pediatrics 2002;110(3):597-608.
(PM:12205266)
[88] Demmers TA, Jones PJ, Wang Y, Krug
S, Creutzinger V, Heubi JE. Effects of early cholesterol intake on cholesterol
biosynthesis and plasma lipids among infants until 18 months of age. Pediatrics
2005;115(6):1594-1601. (PM:15930221)
[89] Bayley TM, Alasmi M, Thorkelson T,
Jones PJ, Corcoran J, Krug-Wispe S, Tsang RC. Longer term effects of early
dietary cholesterol level on synthesis and circulating cholesterol
concentrations in human infants. Metabolism 2002;51(1):25-33.
(PM:11782868)
[90] Mize CE, Uauy R, Kramer R, Benser M,
Allen S, Grundy SM. Lipoprotein-cholesterol responses in healthy infants fed
defined diets from ages 1 to 12 months: comparison of diets predominant in
oleic acid versus linoleic acid, with parallel observations in infants fed a
human milk-based diet. J Lipid Res 1995;36(6):1178-1187.
(PM:7665996)
[91] Lapinleimu H, Viikari J, Jokinen E,
Salo P, Routi T, Leino A, Rönnemaa T, Seppanen R, Valimaki I, Simell O.
Prospective randomized, trial in 1062 infants of diet low in saturated fat and
cholesterol. Lancet 1995;345:471-476.
[92] Simell O, Niinikoski H,
Rönnemaa T, Lapinleimu H, Routi T, Lagström H, Salo P, Jokinen E,
Viikari J. Special Turku Coronary Risk Factor Intervention Project for Babies
(STRIP). Am J Clin Nutr 2000;72(5 Suppl):1316S-1331S.
(PM:11063474)
[93] Rask-Nissilä L, Jokinen E,
Terho P, Tammi A, Lapinleimu H, Rönnemaa T, Viikari J, Seppänen R,
Korhonen T, Tuominen J, Välimäki I, Simell O. Neurological
development of 5-year-old children receiving a low-saturated fat,
low-cholesterol diet since infancy: A randomized controlled trial. JAMA
2000;284(8):993-1000. (PM:10944645)
[94] Kaitosaari T, Rönnemaa T,
Raitakari O, Talvia S, Kallio K, Volanen I, Leino A, Jokinen E,
Välimäki I, Viikari J, Simell O. Effect of 7-year infancy-onset
dietary intervention on serum lipoproteins and lipoprotein subclasses in
healthy children in the prospective, randomized Special Turku Coronary Risk
Factor Intervention Project for Children (STRIP) study. Circulation
2003;108(6):672-677. (PM:12885748)
[95] Svahn JC, Feldl F, Raiha NC,
Koletzko B, Axelsson IE. Different quantities and quality of fat in milk
products given to young children: effects on long chain polyunsaturated fatty
acids and trans fatty acids in plasma. Acta Paediatr 2002;91(1):20-29.
(PM:11885548)
[96] Shannon BM, Tershakovec AM, Martel
JK, Achterberg CL, Cortner JA, Smiciklas-Wright HS, Stallings VA, Stolley PD.
Reduction of elevated LDL-cholesterol levels of 4- to 10-year-old children
through home-based dietary education. Pediatrics 1994;94(6 Pt 1):923-927.
(PM:7971012)
[97] Tershakovec AM, Shannon BM,
Achterberg CL, McKenzie JM, Martel JK, Smiciklas-Wright H, Pammer SE, Cortner
JA. One-year follow-up of nutrition education for hypercholesterolemic
children. Am J Public Health 1998;88(2):258-261.
(PM:9491017)
[98] Kuehl KS, Cockerham JT, Hitchings M,
Slater D, Nixon G, Rifai N. Effective control of hypercholesterolemia in
children with dietary interventions based in pediatric practice. Prev Med
1993;22(2):154-166. (PM:848385)
[99] Tonstad S, Knudtzon J, Sivertsen M,
Refsum H, Ose L. Efficacy and safety of cholestyramine therapy in peripubertal
and prepubertal children with familial hypercholesterolemia. J Pediatr
1996;129(1):42-49. (PM:8757561)
[100] Luepker RV, Perry CL, McKinlay SM,
Nader PR, Parcel GS, Stone EJ, Webber LS, Elder JP, Feldman HA, Johnson CC.
Outcomes of a field trial to improve children's dietary patterns and physical
activity. The Child and Adolescent Trial for Cardiovascular Health. CATCH
collaborative group. JAMA 1996;275(10):768-776.
(PM:8598593)
[101] Niinikoski H, lagstrom H, Jokinen
E, Siltala M, Ronnemaa T, Viikari J, Raitakari OT, Jul;a A, Marniema J,
Nanto-Salonen K, Simell O. Impact of repeated dietary counseling between
infancy and 14 years of age on dietary intakes and serum lipids andf
lipoproteins: the STRIP study. Circulation 2007;116:1032-1040. (PM:
17698729)
[102] Niinikoski H, Koskinen P, Punnonen
K, Seppänen R, Viikari J, Rönnemaa T, Irjala K, Simell O. Intake and
indicators of iron and zinc status in children consuming diets low in saturated
fat and cholesterol: the STRIP baby study. Special Turku Coronary Risk Factor
Intervention Project for Babies. Am J Clin Nutr 1997;66(3):569-574.
(PM:9280175)
[103] Efficacy and safety of lowering
dietary intake of fat and cholesterol in children with elevated low-density
lipoprotein cholesterol. The Dietary Intervention Study in Children (DISC). The
Writing Group for the DISC Collaborative Research Group. JAMA. 1995 May
10;273(18):1429-35. (PM:7723156)
[104] Kwiterovich PO Jr, Barton BA,
McMahon RP, Obarzanek E, Hunsberger S, Simons-Morton D, Kimm SY, Friedman LA,
Lasser N, Robson A, Lauer R, Stevens V, Van Horn L, Gidding S, Snetselaar L,
Hartmuller VW, Greenlick M, Franklin F Jr. Effects of diet and sexual
maturation on low-density lipoprotein cholesterol during puberty: the Dietary
Intervention Study in Children (DISC). Circulation 1997;96(8):2526-2533.
(PM:9355889)
[105] Obarzanek E, Kimm SY, Barton BA,
Van Horn L L, Kwiterovich PO Jr, Simons-Morton DG, Hunsberger SA, Lasser NL,
Robson AM, Franklin FA Jr, Lauer RM, Stevens VJ, Friedman LA, Dorgan JF,
Greenlick MR; DISC Collaborative Research Group. Long-term safety and efficacy
of a cholesterol-lowering diet in children with elevated low-density
lipoprotein cholesterol: seven-year results of the Dietary Intervention Study
in Children (DISC). Pediatrics 2001;107(2):256-264.
(PM:11158455)
[106] Van Horn L, Obarzanek E, Friedman
LA, Gernhofer N, Barton B. Children's adaptations to a fat-reduced diet: the
Dietary Intervention Study in Children (DISC). Pediatrics
2005;115(6):1723-1733. (PM:15930237)
[107] McGowan MP, Joffe A, Duggan AK,
McCay PS. Intervention in hypercholesterolemic college students: a pilot study.
J Adolesc Health 1994;15(2):155-162. (PM:8018689)
[108] Tammi A, Rönnemaa T, Gylling
H, Rask-Nissilä L, Viikari J, Tuominen J, Pulkki K, Simell O. Plant stanol
ester margarine lowers serum total and low-density lipoprotein cholesterol
concentrations of healthy children: the STRIP project. Special Turku Coronary
Risk Factors Intervention Project. J Pediatr 2000;136(4):503-510.
(PM:10753249)
[109] Tammi A, Ronnemaa T, Valsta L,
Seppanen R, Rask-Nissila L, Miettinen TA, Gylling H, Viikari J, Anttolainen M,
Simell O. Dietary plant sterols alter the serum plant sterol concentration but
not the cholesterol precursor sterol concentrations in young children (the
STRIP study). Special Turku Coronary Risk Factor Intervention Project. J Nutr
2001;131:1942-1945. (PM:11435511)
[110] Williams CL, Bollella MC, Strobino
BA, Boccia L, Campanaro L. Plant stanol ester and bran fiber in childhood:
effects on lipids, stool weight and stool frequency in preschool children. J Am
Coll Nutr 1999;18(6):572-581. (PM:10613408)
[111] Ketomaki AM, Gylling H, Antikainen
M, Siimes MA, Miettinen TA. Red cell and plasma plant sterols are related
during consumption of plant stanol and sterol ester spreads in children with
hypercholesterolemia. J Pediatr 2003;142(5):524-531.
(PM:12756385)
[112] Amundsen AL, Ose L, Nenseter MS,
Ntanios FY. Plant sterol ester-enriched spread lowers plasma total and LDL
cholesterol in children with familial hypercholesterolemia. Am J Clin Nutr
2002;76(2):338-344. (PM:12145004)
[113] Jakulj L, Vissers MN, Rodenburg J,
Wiegman A, Trip MD, Kastelein JJ. Plant stanols do not restore endothelial
function in pre-pubertal children with familial hypercholesterolemia despite
reduction of low-density lipoprotein cholesterol levels. J Pediatr
2006;148(4):495-500. (PM:16647412)
[114] Gylling H, Siimes MA, Miettinen TA.
Sitostanol ester margarine in dietary treatment of children with familial
hypercholesterolemia. J Lipid Res 1995;36(8):1807-1812.
(PM:7595101)
[115] de Jongh S, Vissers MN, Rol P,
Bakker HD, Kastelein JJ, Stroes ES. Plant sterols lower LDL cholesterol without
improving endothelial function in prepubertal children with familial
hypercholesterolaemia. J Inherit Metab Dis 2003;26(4):343-351.
(PM:12971422)
[116] McCrindle BW, Helden E, Conner WT.
Garlic extract therapy in children with hypercholesterolemia. Arch Pediatr
Adolesc Med 1998;152(11):1089-1094. (PM:9811286)
[117] Laurin D, Jacques H, Moorjani S,
Steinke FH, Gagné C, Brun D, Lupien PJ. Effects of a soy-protein
beverage on plasma lipoproteins in children with familial hypercholesterolemia.
Am J Clin Nutr 1991;54(1):98-103. (PM:2058593)
[118] Engler MM, Engler MB, Malloy M,
Chiu E, Besio D, Paul S, Stuehlinger M, Morrow J, Ridker P, Rifai N,
Mietus-Snyder M. Docosahexaenoic acid restores endothelial function in children
with hyperlipidemia: results from the EARLY study. Int J Clin Pharmacol Ther
2004;42(12):672-679. (PM:15624283)
[119] Williams CL, Bollella M, Spark A,
Puder D. Soluble fiber enhances the hypocholesterolemic effect of the step I
diet in childhood. J Am Coll Nutr 1995;14(3):251-257. (PM:
8586774)
[120] Davidson MH, Dugan LD, Burns JH,
Sugimoto D, Story K, Drennan K. A psyllium-enriched cereal for the treatment of
hypercholesterolemia in children: a controlled, double-blind, crossover study.
Am J Clin Nutr 1996;63(1):96-102. (PM:8604676)
[121] Dennison BA, Levine DM. Randomized,
double-blind, placebo-controlled, two-period crossover clinical trial of
psyllium fiber in children with hypercholesterolemia. J Pediatr
1993;123(1):24-29. (PM:8391569)
[122] Engler MM, Engler MB, Malloy MJ,
Chiu EY, Schloetter MC, Paul SM, Stuehlinger M, Lin KY, Cooke JP, Morrow JD,
Ridker PM, Rifai N, Miller E, Witztum JL, Mietus-Snyder M. Antioxidant vitamins
C and E improve endothelial function in children with hyperlipidemia:
Endothelial Assessment of Risk from Lipids in Youth (EARLY) Trial. Circulation
2003;108(9):1059-1063. (PM:12912807)
[123] Epstein LH, Kuller LH, Wing RR,
Valoski A, McCurley J. The effect of weight control on lipid changes in obese
children. Am J Dis Child 1989;143(4):454-457. (PM:2929526)
[124] Nemet D, Barkan S, Epstein Y,
Friedland O, Kowen G, Eliakim A. Short- and long-term beneficial effects of a
combined dietary-behavioral-physical activity intervention for the treatment of
childhood obesity. Pediatrics 2005;115(4):e443-e449.
(PM:15805347)
[125] Kang HS, Gutin B, Barbeau P, Owens
S, Lemmon CR, Allison J, Litaker MS, Le NA. Physical training improves insulin
resistance syndrome markers in obese adolescents. Med Sci Sports Exerc
2002;34(12):1920-1927. (PM:12471297)
[126] Becque MD, Katch VL, Rocchini AP,
Marks CR, Moorehead C. Coronary risk incidence of obese adolescents: reduction
by exercise plus diet intervention. Pediatrics 1988;81(5):605-612.
(PM:3357722)
[127] Ferguson MA, Gutin B, Le NA, Karp
W, Litaker M, Humphries M, Okuyama T, Riggs S, Owens S. Effects of exercise
training and its cessation on components of the insulin resistance syndrome in
obese children. Int J Obes Relat Metab Disord 1999;23(8):889-895.
(PM:10490792)
[128] Jacques H, Laurin D, Moorjani S,
Steinke FH, Gagné C, Brun D, Lupien PJ. Influence of diets containing
cow's milk or soy protein beverage on plasma lipids in children with familial
hypercholesterolemia. J Am Coll Nutr 1992;11 Suppl:69S-73S.
(PM:1619204)
[129] Pieke B, von Eckardstein A,
Gulbahce E, Chirazi A, Schulte H, Assmann G, Wahrburg U. Treatment of
hypertriglyceridemia by two diets rich in unsaturated fatty acids or in
carbohydrates: effects on lipoprotein subclasses, lipolytic enzymes, lipid
transfer proteins, insulin and leptin. Int J of Obesity 2000;24:1286-1296.
[130] Ohta T, Nakamura R, Ikeda Y,
Hattori S, Matsuda I. Follow up study on children with dyslipidaemia detected
by mass screening at 18 months of age: effect of 12 months dietary treatment.
Eur J Pediatr 1993;152(11):939-943.
[131] Pan Y, Pratt CA. Metabolic syndrome
and its association with diet and physical activity in US adolescents. J Am
Diet Assoc 2008;108(2):276-286.
[132] Pereira MA, Swain J, Goldfine AB,
Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy
expenditure and heart disease risk factors during weight loss. JAMA. 2004 Nov
24;292(20):2482-90. (PM:15562127)
[133] Ebbeling CB, Leidig MM, Sinclair
KB, Seger-Shippee LG, Feldman HA, Ludwig DS. Effects of an ad libitum
low-glycemic load diet on cardiovascular disease risk factors in obese young
adults. Am J Clin Nutr. 2005 May;81(5):976-82.
(PM:15883418)
[134] Ebbeling CB, Leidig MM, Feldman HA,
Lovesky MM, Ludwig DS. Effects of a low glycemic-load vs low fat diet in obese
young adults. JAMA 2007;297:2092-2102. (PM: 17507345)
[135] Ebbeling CB, Leidig MM, Sinclair
KB, Hangen JP, Ludwig DS. A reduced glycemic load diet in the treatment of
adolescent obesity. Arch Pediatr Adolesc med 2003;157:773-779. (PM:
12912783)
[136] Sondike SB, Copperman N, Jacobson
MS. Effects of a low-carbohydrate diet on weight loss and cardiovascular risk
factors in overweight adolescents. J Pediatr 2003;142(3):253-258.
(PM:12640371)
[137] McCrindle BW, Urbina EM, Dennison
BA, Jacobson MS, Steinberger J, Rocchini AP, Hayman LL, Daniels SR, American
Heart Association Atherosclerosis, Hypertension, and Obesity in Youth
Committee, American Heart Association Council of Cardiovascular Disease in the
Young, American Heart Association Council on Cardiovascular Nursing. Drug
therapy of high-risk lipid abnormalities in children and adolescents: a
scientific statement from the American Heart Association Atherosclerosis,
Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease
in the Young, with the Council on Cardiovascular Nursing.
Circulation 2007;115(14):1948-67. (PM:17377073)
[138] Daniels SR, Greer FR, Committee on
Nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics
2008;122(1):198-208. (PM:18596007)
[139] McCrindle BW, Ose L, Marais AD.
Efficacy and safety of atorvastatin in children and adolescents with familial
hypercholesterolemia or severe hyperlipidemia: a multicenter, randomized,
placebo-controlled trial. J Pediatr 2003;143(1):74-80.
(PM:12915827)
[140] van der Graaf A, Nierman MC, Firth
JC, Wolmarens KH, Marais AD, de Groot E. Efficacy and safety of fluvastatin in
children and adolescents with heterozygous familial hypercholesterolemia. Acta
Paediatrica 2006; 95:1461-1466.
[141] Lambert M, Lupien PJ, Gagné
C, Lévy E, Blaichman S, Langlois S, Hayden M, Rose V, Clarke JT, Wolfe
BM, Clarson C, Parsons H, Stephure DK, Potvin D, Lambert J. Treatment of
familial hypercholesterolemia in children and adolescents: effect of
lovastatin. Canadian Lovastatin in Children Study Group. Pediatrics
1996;97(5):619-628. (PM:8628597)
[142] Stein EA, Illingworth DR,
Kwiterovich PO Jr, Liacouras CA, Siimes MA, Jacobson MS, Brewster TG, Hopkins
P, Davidson M, Graham K, Arensman F, Knopp RH, DuJovne C, Williams CL,
Isaacsohn JL, Jacobsen CA, Laskarzewski PM, Ames S, Gormley GJ. Efficacy and
safety of lovastatin in adolescent males with heterozygous familial
hypercholesterolemia: a randomized controlled trial. JAMA 1999;281(2):137-144.
(PM:9917116)
[143] Clauss SB, Holmes KW, Hopkins P,
Stein E, Cho M, Tate A, Johnson-Levonas AO, Kwiterovich PO. Efficacy and safety
of lovastatin therapy in adolescent girls with heterozygous familial
hypercholesterolemia. Pediatrics 2005;116(3):682-688.
(PM:16140708)
[144] Knipscheer HC, Boelen CC, Kastelein
JJ, van Diermen DE, Groenemeijer BE, van den Ende A, Büller HR, Bakker HD.
Short-term efficacy and safety of pravastatin in 72 children with familial
hypercholesterolemia. Pediatr Res 1996;39(5):867-871.
(PM:8726243)
[145] de Jongh S, Ose L, Szamosi T,
Gagné C, Lambert M, Scott R, Perron P, Dobbelaere D, Saborio M, Tuohy
MB, Stepanavage M, Sapre A, Gumbiner B, Mercuri M, van Trotsenburg AS, Bakker
HD, Kastelein JJ; Simvastatin in Children Study Group. Efficacy and safety of
statin therapy in children with familial hypercholesterolemia: a randomized,
double-blind, placebo-controlled trial with simvastatin. Circulation
2002;106(17):2231-2237. (PM:12390953)
[146] McCrindle BW, O'Neill MB,
Cullen-Dean G, Helden E. Acceptability and compliance with two forms of
cholestyramine in the treatment of hypercholesterolemia in children: a
randomized, crossover trial. J Pediatr 1997;130(2):266-273.
(PM:9042130)
[147] Tonstad S, Sivertsen M, Aksnes L,
Ose L. Low dose colestipol in adolescents with familial hypercholesterolaemia.
Arch Dis Child 1996;74(2):157-160. (PM:8660081)
[148] McCrindle BW, Helden E, Cullen-Dean
G, Conner WT. A randomized crossover trial of combination pharmacologic therapy
in children with familial hyperlipidemia. Pediatr Res 2002;51(6):715-721.
(PM:12032266)
[149] Colletti RB, Neufeld EJ, Roff NK,
McAuliffe TL, Baker AL, Newburger JW. Niacin treatment of hypercholesterolemia
in children. Pediatrics 1993;92:78-82.
[150] Wheeler KA, West RJ, Lloyd JK,
Barley J. Double blind trial of bezafibrate in familial hypercholesterolaemia.
Arch Dis Child 1985;60(1):34-37. (PM:3882058)
[151] Avis HJ, Hutten BA, Gagne C,
Langslet G, McCrindle BW, Wiegman A, et al. Efficacy and safety of rosuvastatin
therapy for children with familial hypercholesterolemia. J Am Coll Cardiol.
2010;55:1121-6.
[152] Avis HJ, Vissers MN, Stein EA,
Wijburg FA, Trip MD, Kastelein JJP, Hutten BA. A systematic review and
meta-analysis of statin therapy in children with familial hypercholesterolemia.
Arterioscle Thromb Vasc Biol 2007;27:1803-1810.
(PM:17569881)
[153] Stein EA, Marais AD, Szamosi T,
Raal FJ, Schurr D, Urbina EM, Hopkins PN, Karki S, Xu J, Misir S, Melino M.
Colesevelam hydrochloride: efficacy and safety in pediatric subjects with
heterozygous familial hypercholesterolemia. J Pediatr 2010;156(2):231-6.
154 van der Graaf A, Cuffie-Jackson C, Vissers MN, Trip
MD, Gagne C, Shi G, Veltri E, Avis HJ, Kastelein JJP. Efficacy and safety
of coadministration of ezetimibde and simvastatin in adolescents with
heterozygous familial hypercholesterolemia. J Amer Coll Cardiol
2008;52:1421-1429.
[155] Gagne C, Gaudet D, Bruckert E;
Ezetimibde Study group. Efficacy and safety of ezetimibde coadministration with
atorvastatin or simvastatin in patients with homozygous familial
hypercholesterolemia. Circulation 2002;105:2469-2475.
[156] Goldberg RB, Sabharal AK. Fish oil
in the treatment of dyslipidemia. Current Opin Endocrinol Diabetes and Obes
2008;15:167-174.
[157] Engler MM, Engler MB, Malloy MJ,
Paul SM, Kulkarni KR, Mietus-Snyder ML. Effect of docosahexaenoic acid on
lipoprotein subclasses in hyperlipidemic children (the EARLY study). Am J
Cardiol 2005;95(7):869-871. (PM:15781019)
[158] Tonstad S, Aksnes L. Fat-soluble
vitamin levels in familial hypercholesterolemia. J Pediatr
1997;130:274-280.
Back to Top
Back to Table
of Contents
|
 |