ClinicalTrials.gov to Include Basic Results Data
E ffective late September 2008, basic results data for certain clinical trials are required to be submitted by the sponsor or designated principal investigator to ClinicalTrials.gov for public posting. Some basic results for clinical trials will be submitted in late September; over time, increasing numbers of basic results submissions will be made available to the public. This reporting requirement is mandated by Section 801 of the Food and Drug Administration Amendments Act of 2007 (FDAAA 801)]. In general, FDAAA 801 expands the scope of trials and information required to be submitted to ClinicalTrials.gov and requires submission of results data in several steps.
ClinicalTrials.gov was modified in November 2007 to accommodate the first step of FDAAA 801, which included:
- expanding the scope of required trials to include studies of devices and all diseases (only trials of drugs and biologics for serious or life-threatening diseases or conditions were required to be registered previously); and
- increasing the information required to be registered (e.g., the primary and secondary outcome measures being studied).
The second step involved accepting basic results information and making it publicly available. The summary data tables required for reporting trial results include the following:
- baseline characteristics, which are taken at the beginning of a trial and may include demographic and physiologic characteristics of the participants;
- participant flow to indicate the number of participants at each stage of the trial; and
- outcomes data, including pre-specified primary and secondary outcomes, and relevant statistical analyses.
Submission of adverse events information is not required by law until September 2009; however, optional tables for reporting serious adverse events and other, frequent adverse events observed during the trial are available now. The ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT) document includes definitions and annotations describing the minimal requirements for reporting results to ClinicalTrials.gov. Only those trials that are registered with ClinicalTrials.gov will be able to report results.
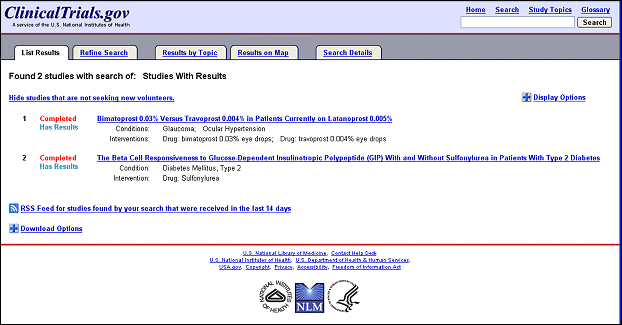
Several changes to the user interface were made to facilitate the availability of and search for basic results on ClinicalTrials.gov. The design of the basic results page is based on the look and feel of the updated user interface released in September 2007 (Williams RJ, Tse T. New Look for ClinicalTrials.gov. NLM Tech Bull. 2007 Sep-Oct; (358):e2.). Studies with results available are identified on the List Results page by a new term, "Has results" (see Figure 1).
The study record features a new tab entitled either "No Study Results Posted" (in gray) or "Study Results" (in blue), depending on the availability of results. If no results are posted for a study, selecting the "grayed out" tab displays the following statement, "No Study Results Posted for this Study," and explanatory information about why results may not be available (e.g., trial not yet completed, mandatory submission requirements do not apply). If results have been posted, clicking on the "Study Results" tab will display those basic results data. A mock-up of the basic results display template is presented in Figure 2. See the ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT) document to learn about all information that may be posted on the Study Results page.
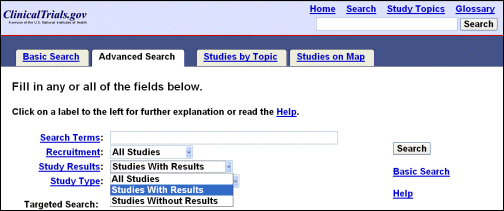
The other significant change to the user interface is a new feature in "Advanced Search" titled "Study Results" (see Figure 3). Users can now limit a search based on the availability of results (i.e., all studies, studies with results, studies without results).
While the initial implementation of the Basic Results data entry and display features at ClinicalTrials.gov are now available, there are additional steps to be addressed. For example, another step defined by the law requires issuing new regulations to expand the results requirements. In addition, new search features, links to relevant resources, and other capabilities to facilitate users' information seeking needs will be developed. To receive occasional e-mail announcements about the latest changes to ClinicalTrials.gov, join the NIH FDAAA-UPDATE-L Listserv.
Contact Information
Please send your comments or questions to: custserv@nlm.nih.gov.
Tse T, Williams RJ. ClinicalTrials.gov to Include Basic Results Data. NLM Tech Bull. 2008 Sep-Oct; (364):e15.