
The Division of Intramural Research conducts a broad program of laboratory and clinical research.

 An OverviewAbout DIR, mission, vision, values
An OverviewAbout DIR, mission, vision, values Branches
Branches

 Research InvestigatorsScientist profiles, publications
Research InvestigatorsScientist profiles, publications Clinical ResearchCurrent clinical studies, FAQ
Clinical ResearchCurrent clinical studies, FAQ NHGRI Affiliated CentersCIDR, NCATS, NISC, CRGGH
NHGRI Affiliated CentersCIDR, NCATS, NISC, CRGGH Online Research ResourcesDatabases, software, tools
Online Research ResourcesDatabases, software, tools DIR CalendarWorkshops, conferences, genome seminar series, courses
DIR CalendarWorkshops, conferences, genome seminar series, courses Publications, Books and Resources
Publications, Books and Resources Organizational ChartFor the Division of Intramural Research
Organizational ChartFor the Division of Intramural Research
Feature
FDA approves crystal-dissolving eye drops, a major milestone for NIH rare disease researchers
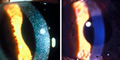 Read the heroic story about how an experimental eye-drop solution containing a drug called cysteamine eliminates the the painful and destructive crystalline shards in the eyes of patients suffering from a rare, inherited condition called nephropathic cystinosis. Learn how the eyedrop went from clinical trial, to FDA approval and availability for use on Oct. 3, 2012, The research was spearheaded by NHGRI Clinical Director, William Gahl, M.D., Ph.D. (more)
Read the heroic story about how an experimental eye-drop solution containing a drug called cysteamine eliminates the the painful and destructive crystalline shards in the eyes of patients suffering from a rare, inherited condition called nephropathic cystinosis. Learn how the eyedrop went from clinical trial, to FDA approval and availability for use on Oct. 3, 2012, The research was spearheaded by NHGRI Clinical Director, William Gahl, M.D., Ph.D. (more)
See Also: DIR News Features
Current Topics in Genome Analysis 2012
Current Topics in Genome Analysis lecture series consists of lectures by local and outside speakers covering the major areas of genomics.
Highlights
NHGRI and JHU scientists collaborate on mechanized analysis of melanocyte regulators
 A team that includes Stacie Loftus, Ph.D., and William Pavan, Ph.D.
A team that includes Stacie Loftus, Ph.D., and William Pavan, Ph.D., both from NHGRI's Genetic Diseases Research Branch, and colleagues from the Johns Hopkins University, report in the Sept. 27, 2012, early online issue of
Genome Research their research to detect regulators in mouse and human melanocytes. The team used an automated approach to the investigation of regulatory sequences.
Read the study:
Integration of ChIP-seq and machine learning reveals enhancers and a predictive regulatory sequence vocabulary in melanocytes [genome.cshlp.org]
Recent Studies and Papers
Last Updated: September 28, 2012







 An Overview
An Overview Branches
Branches
 Cancer Genetic Branch
Cancer Genetic Branch Inherited Disease Research Branch
Inherited Disease Research Branch Genetic Disease Research Branch
Genetic Disease Research Branch Medical Genetics Branch
Medical Genetics Branch Genetics and Molecular Biology Branch
Genetics and Molecular Biology Branch Social and Behavioral Research Branch
Social and Behavioral Research Branch Genome Technology Branch
Genome Technology Branch Research Investigators
Research Investigators Clinical Research
Clinical Research NHGRI Affiliated Centers
NHGRI Affiliated Centers Online Research Resources
Online Research Resources DIR Calendar
DIR Calendar Publications, Books and Resources
Publications, Books and Resources Organizational Chart
Organizational Chart




