Principles of HCT
Autologous and Allogeneic HCT
Determining when HCT is indicated: Comparison of HCT and chemotherapy outcomes
HLA matching and hematopoietic stem cell sources
Autologous and Allogeneic HCT
The two major transplant approaches currently in use include autologous (using the patient's own hematopoietic stem cells) and allogeneic (using related or unrelated donor hematopoietic stem cells). Autologous transplant treats cancer by exposing patients to mega-dose (myeloablative) therapy with the intent of overcoming chemotherapy resistance in tumor cells, followed by infusion of the patient’s previously stored hematopoietic stem cells. In order for this approach to work, the following must apply:
- The higher chemotherapy/radiation therapy dose that can be used because of hematopoietic stem cell support achieves a significantly higher cell kill of the disease. This may include increased tumor kill in areas where standard-dose chemotherapy has less penetration (CNS).
- Meaningful percentages of cure or long-term remission from the disease must occur without significant nonhematopoietic toxicities limiting the therapeutic benefit achieved.
Allogeneic approaches to cancer may involve high-dose therapy as well, but because of immunologic differences between the donor and recipient, an additional graft-versus-tumor (GVT) or graft-versus-leukemia (GVL) treatment effect can occur. Although autologous approaches are associated with less short-term mortality, many malignancies are resistant to mega-dose therapy alone and/or involve the bone marrow, thus requiring allogeneic approaches for optimal outcome.
Determining when HCT is indicated: Comparison of HCT and chemotherapy outcomesBecause the outcomes using chemotherapy and hematopoietic cell transplantation (HCT) treatments tend to improve with time, regular comparisons should be performed between these approaches to continually redefine optimal therapy for a given patient. For some diseases, randomized trials or intent-to-treat using a human leukocyte antigen (HLA)-matched sibling donor have established the benefit of HCT by direct comparison.[1,2] However, for very high-risk patients such as those with early relapse of ALL, randomized trials have not been feasible due to investigator bias.[3,4] In general, HCT approaches only offer benefit to children at high risk for relapse with standard chemotherapy approaches; therefore, treatment schemes that accurately identify high-risk patients and offer HCT if reasonably HLA-matched donors are available, saving higher risk approaches to HCT (HLA haploidentical or significantly mismatched donors) for only the very highest risk patients, has come to be the preferred approach for many diseases.[5]
When comparisons of similar patients treated with HCT or chemotherapy are made and randomized or intent-to-treat studies are not feasible, the following issues should be considered:
- Remission status: Because patients failing to obtain remission do very poorly with any therapy, comparisons between HCT and chemotherapy should include only those who obtain remission, preferably after similar approaches to salvage therapy. In order to account for time-to-transplant bias, the chemotherapy comparator arm should only include patients who maintained remission until the median time to HCT. The HCT comparator arm should also only include patients who achieved the initial remission mentioned above and maintained that remission until the time of HCT.
- Past or current therapy approaches used: Disease- and era-appropriate chemotherapy and HCT approaches should be compared.
- HCT approach: HCT approaches that are very high risk or have documented lower rates of survival (i.e., haploidentical approaches) should not be combined for analysis with standard-risk HCT approaches (matched sibling and well-matched unrelated donors).
- Criteria for relapse: Risk factors for relapse should be carefully defined and analysis based upon the most current knowledge of risk should be performed. One should avoid combining high- and intermediate-risk patient groups because a benefit for HCT in the high-risk group can be masked when outcomes are similar in the intermediate-risk group.[6]
- Selection Bias: Attempts should be made to understand and eliminate or correct for selection bias. Examples include the following:
- Higher risk patients preferentially undergoing HCT (i.e., patients who take several rounds to achieve remission or who relapse after obtaining remission and go back into a subsequent remission prior to HCT).
- Sicker patients deferred from HCT because of comorbidities.
- Patient/parent refusal.
- Lack of or inability to obtain insurance approval for HCT.
- Lack of access to HCT due to distance/inability to travel.
One source of bias difficult to control for or detect is physician bias for or against HCT. The effect of access to HCT and therapeutic bias on outcomes of pediatric malignancies where HCT may be indicated has been poorly studied to date.
HLA matching and hematopoietic stem cell sourcesHLA overview
Appropriate matching between donor and recipient HLA in the major histocompatibility complex located on chromosome 6 is essential to successful allogeneic HCT (see Table 1).
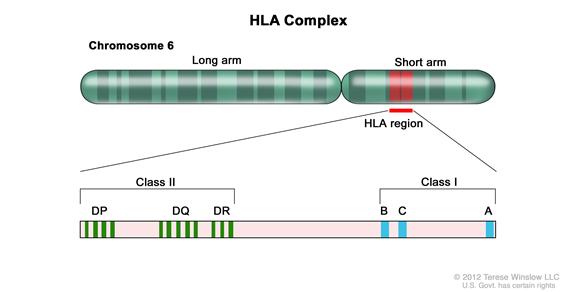
HLA class I (A, B, C, etc.) and class II (DRB1, DQB1, DPB1, etc.) alleles are highly polymorphic, therefore finding appropriately matched unrelated donors is a challenge for some patients, especially those of certain racial groups (e.g., African Americans and Hispanics).[7] Because full siblings of cancer patients have a 25% chance of being HLA matched, they have been the preferred source of allogeneic hematopoietic stem cells. Early serologic techniques of HLA assessment defined a number of HLA antigens, but more precise DNA methodologies have shown HLA allele-level mismatches in up to 40% of serologic HLA antigen matches. These differences are clinically relevant, as use of donors with allele-level mismatches affect survival and rates of graft-versus-host disease (GVHD) to a degree similar to patients with antigen-level mismatches.[8] Because of this, DNA-based allele-level HLA typing is standard when choosing unrelated donors.
Table 1. Level of Human Leukocyte Antigen (HLA) Typing Currently Used for Different Hematopoietic Stem Cell Sourcesa,b| Class I Antigens | Class II Antigens | |||||
| Stem Cell Source | HLA A | HLA B | HLA C | HLA DRB1 | HLA DQB1 | HLA DPB1 |
| Matched Sibling BM/PBSCs | Antigen | Antigen | Optional | Allele | ||
| Matched Sibling/Other Related Donorc BM/PBSCs | Allele | Allele | Allele | Allele | Optional | Optional |
| Unrelated Donor BM/PBSCs | Allele | Allele | Allele | Allele | Optional | Optional |
| Unrelated Cord Blood | Antigen (Allele Optional) | Antigen (Allele Optional) | Allele Optional | Allele | Optional | Optional |
| BM = bone marrow; PBSC = peripheral blood stem cells. | ||||||
| aHLA antigen: A serologically defined, low-resolution method of defining an HLA protein. Differs from allele-level typing half of the time. Designated by the first two numbers (i.e., HLA B 35:01—antigen is HLA B 35). | ||||||
| bHLA allele: A higher resolution method of defining unique HLA proteins by typing their gene through sequencing or other DNA-based methods that detect unique differences. Designated by at least four numbers (i.e., HLA B 35:01). | ||||||
| cParent, cousin, etc., with a phenotypic match or near-complete HLA match. |
Table 2. Definitions of the Numbers Describing Human Leukocyte Antigen (HLA) Antigens and Alleles Matching
| If These HLA Antigens and Alleles Match | Then the Donor is Considered to be This Type of Match |
| A, B, and DRB1 | 6/6 |
| A, B, C, and DRB1 | 8/8 |
| A, B, C, DRB1, and DQB1 | 10/10 |
| A, B, C, DRB1, DQB1, and DPB1 | 12/12 |
HLA matching considerations for sibling and related donors
The most commonly used related donor is a sibling from the same parents who is HLA matched for HLA A, HLA B, and HLA DRB1 at a minimum, at the antigen level. Given the distance on chromosome 6 between HLA A and HLA DRB1, there is approximately a 1% possibility of a crossover event occurring in a possible sibling match. Because a crossover event could involve the HLA C antigen and because parents may share HLA antigens that actually differ at the allele level, many centers perform allele-level typing of possible sibling donors at all of the key HLA antigens (HLA A, B, C, and DRB1). Any related donor that is a nonfull sibling should have full HLA typing because similar haplotypes from different parents could differ at the allele level. Although single-antigen mismatched related donors (5/6 antigen matched) have been used interchangeably with matched sibling donors in some studies in the past, a large Center for International Blood and Marrow Transplant Research (CIBMTR) study in pediatric HCT recipients showed that use of 5/6 antigen matched related donors who are not siblings results in rates of GVHD and overall survival equivalent to 8/8 allele level matched unrelated donors and slightly inferior survival compared to fully matched siblings.[9]
HLA matching considerations for unrelated donorsOptimal outcomes are achieved in unrelated allogeneic marrow transplantation when the pairs of antigens at HLA A, B, C, and DRB1 are matched between the donor and the recipient at the allele level (termed an 8/8 match).[10] A single antigen/allele mismatch at any of these antigens (7/8 match) lowers the probability of survival between 5% to 10% with a similar increase in the amount of significant (grades III–IV) acute GVHD.[10] Of these four antigen pairs, different reports have shown HLA A, C, and DRB1 mismatches to potentially be more highly associated with mortality than the other antigens,[8,10,11] but the differences in outcome are small and inconsistent, making it very difficult to conclude presently that one can pick a more favorable mismatch by choosing one type of antigen mismatch above another. Many groups are attempting to define specific antigens or pairs of antigens that are associated with poor outcome, but such studies require very large numbers of patients and exclusion of specific antigens or antigen pairs for donor choice is not widely practiced.
Although it is well understood that class II antigen DRB1 mismatches increase GVHD and worsen survival,[11] the need to match for two other important class II antigens, DQB1 and DPB1, is controversial. DQB1 mismatches have been associated with significant increases in acute GVHD,[12] but subsequent studies have not shown a difference in overall survival.[10] Many centers have adopted a policy to attempt to match patients at DQB1 in addition to the other four pairs of antigens; full matches using this approach are thus termed 10/10 HLA matches. Such matching is possible for a high percentage of patients because of strong linkage disequilibrium between DRB1 and DQB1, resulting in many 8/8 matched donors also being 10/10 matches. DPB1 mismatches have similarly been shown to lead to increased GVHD without a change in survival.[13,14] Although some centers attempt to match for DPB1 (12/12 match), it is challenging, because due to less linkage disequilibrium, only about 15% of 10/10 matches will also be 12/12 matches; studies showing whether it is better to mismatch at DQB1 compared with DPB1 have not been performed. A study grouping DPB1 antigens into permissive groups allowed up to 50% of patients with 10/10 matches to choose a favorable DPB1 match,[15] but this classification system is not yet generally accepted.
HLA matching and cell dose considerations for unrelated cord blood HCTAnother commonly used hematopoietic stem cell source is that of unrelated umbilical cord blood, which is harvested from donor placentas moments after birth and cryopreserved, HLA typed, and banked. Unrelated cord blood transplantation has been successful with less stringent HLA matching requirements compared with standard related or unrelated donors, probably due to limited antigen exposure experienced in utero and different immunological composition. Cord blood matching is generally performed at an intermediate level for HLA A and B and at an allele level (high resolution) for DRB1. This means that matching of only 6 antigens is necessary to choose units for transplantation. Although better outcomes occur when 6/6 or 5/6 HLA matched units are used,[16] successful HCT has occurred even with 4/6 or less matched units in many patients. Better matching of units (matching for HLA-C, as well as A, B, and DRB1) has been shown to result in less transplant-related mortality, but has not been shown to have an impact on survival in children with malignancies.[17] Many centers will type up to 10 alleles and use the best match possible, but the impact of DQB1 mismatched has not been shown to affect outcome. Higher cell doses in cord blood units have been shown to improve outcomes, especially when the units have higher levels of HLA mismatch. One study showed that survival of recipients of 4/6 matched cords with cell doses greater than 5 × 107 total nucleate cells/kg recipient weight is similar to 5/6 matched cord recipients receiving a cell dose of 2.5 to 5 × 107 total nucleate cells/kg. Although no clear improvement in survival occurred for cell doses above 5 × 107 total nucleate cells/kg, higher doses of cells improved outcomes for all levels of HLA mismatch.[18]
In addition to cell dose and matching level, the direction of mismatches between donor and recipient has been shown to be important in cord blood transplant outcomes. When cord blood units have paired identical antigens (for example, both HLA A antigens are HLA A 02), and if the recipient has one HLA A 02 antigen and a second antigen that is mismatched, T-cells from the recipient do not detect a mismatch in the cord and are less likely to reject this cord. T-cells from the cord blood unit in this case, however, would detect the other (non-HLA A 02) mismatched antigen in the A locus of the recipient, potentially stimulating a GVHD reaction (termed mismatch in the GVH direction only [GVH-O]). If the paired identical antigens are in the recipient and not in the cord blood unit, the mismatch is in the rejection direction only (R-O). It has been shown that mismatches that are only in the GVHD direction (GVH-O, paired antigens in the cord blood unit) lead to lower transplant-related mortality and overall mortality, compared with those with R-O mismatches. R-O mismatches have outcomes similar to patients who have bidirectional mismatches.[19] Current recommendations are for transplant centers to give priority to GVH-O mismatches above other mismatches and avoid selecting R-O mismatches.
Two aspects of umbilical cord blood HCT have made it more widely applicable. First, because a successful procedure can occur with multiple HLA mismatches, over 90% of patients from a wide variety of ethnicities are able to find a at least a 4/6 matched cord blood unit, allowing a method of performing HCT for populations that traditionally have not had HCT options because of having rare HLA haplotypes.[7,20] Second, as mentioned above, adequate cell dose (minimum 2–3 × 107 total nucleate cells/kg and 1.7 × 105 CD34+ cells/kg) has been shown to be associated with improved survival.[21,22] Total nucleate cells is generally used to judge units because techniques to measure CD34+ doses have not been standardized. Because even large single umbilical cord blood units are only able to supply these minimum doses to recipients weighing up to 40 kg to 50 kg, early umbilical cord blood HCT focused mainly on smaller children. Later studies showed that this size barrier could be overcome by using two umbilical cord blood units as long as each of the units is at least a 4/6 HLA match with the recipient; because two cords result in much higher cell doses, umbilical cord blood transplantation is now used widely for larger children and adults.[23] Single-center studies have suggested that the use of two umbilical cord blood units may decrease relapse in patients with malignancies; however, this has not been validated in multicenter studies.[24] It has been shown that grades II to IV acute GVHD is higher when two versus one umbilical cord blood unit is used; but transplant-related mortality has not been noted to be increased.[25] One study comparing adult and older pediatric patients transplanted with either double 4/6 to 6/6 matched umbilical cord blood or unrelated bone marrow/PBSCs showed survival to be equivalent.[26]
Comparison of stem cell productsCurrently, the three stem cell products used from both related and unrelated donors are bone marrow, peripheral blood stem cells (PBSCs), and cord blood. In addition, bone marrow or PBSCs can be T-cell depleted by several methods and the resultant stem cell product has very different properties. Finally, partially HLA-matched (half or more antigens [haploidentical]) related bone marrow or PBSCs can be used after in vitro or in vivo T-cell depletion and this product also behaves differently compared with other stem cell products. A comparison of stem cell products is presented in Table 3.
Table 3. Comparison of Hematopoietic Stem Cell Products| PBSCs | BM | Cord Blood | T-cell Depleted BM/PBSCs | Haploidentical T-cell Depleted BM/PBSCs | |
| T-cell content | High | Moderate | Low | Very low | Very low |
| CD34+ content | Moderate–high | Moderate | Low (but higher potency) | Moderate–high | Moderate–high |
| Time to neutrophil recovery | Rapid (13–25 d) | Moderate (15–25 d) | Slow (16–55 d) | Moderate (15–25 d) | Moderate (15–25 d) |
| Early post-HCT risk of infections, EBV-LPD | Low–moderate | Moderate | High | Very High | Very High |
| Risk of graft rejection | Low | Low–moderate | Moderate–high | Moderate–high | Moderate–high |
| Time to immune reconstitutiona | Rapid (6–12 mo) | Moderate (6–18 mo) | Slow (6–24 mo) | Slow (6–24 mo) | Slow (9–24 mo)b |
| Risk of acute GVHD | Moderate | Moderate | Moderate | Low | Low |
| Risk of chronic GVHD | High | Moderate | Low | Low | Low |
| BM = bone marrow; EBV-LPD = Epstein-Barr virus–associated lymphoproliferative disorder; GVHD = graft-versus-host disease; HCT = hematopoietic cell transplantation; PBSCs = peripheral blood stem cells. | |||||
| aAssuming no development of GVHD. If patients develop GVHD, immune reconstitution is delayed until resolution of the GVHD and discontinuation of immune suppression. | |||||
| bIf a haploidentical donor is used, longer times to immune reconstitution may occur. |
The main differences between the products are associated with the numbers of T-cells and CD34+ progenitor cells present; very high levels of T-cells are present in PBSCs, intermediate numbers in bone marrow, and low numbers in cord blood and T-cell depleted products. Patients receiving T-cell depleted products or cord blood generally have slower hematopoietic recovery, increased risk of infection, late immune reconstitution, higher risks of nonengraftment, and increased risk of Epstein-Barr virus (EBV)–associated lymphoproliferative disorder. This is countered by lower rates of GVHD and an ability to offer transplantation to patients where full HLA matching is not available. Higher doses of T-cells in PBSCs result in rapid neutrophil recovery and immune reconstitution, but suffer from increased rates of chronic GVHD.
There are only a few studies directly comparing outcomes of different stem cell sources/products in pediatric patients. A retrospective registry study of pediatric patients undergoing HCT for acute leukemia compared those receiving related donor bone marrow with related donor PBSCs. Although the bone marrow and PBSC recipient cohorts differed some in their risk profiles, after statistical correction, increased risk of GVHD and transplant-related mortality associated with PBSC led to poorer survival in the PBSC group.[27] This report, combined with lack of prospective studies comparing bone marrow and PBSCs, has led most pediatric transplant protocols to prefer bone marrow to PBSCs from related donors. For those requiring unrelated donors, a large Blood and Marrow Transplant Clinical Trials Network (BMT CTN) trial randomizing bone marrow and PBSCs that included pediatric patients has recently completed and analysis of the outcomes will be forthcoming.[28] In an attempt to determine whether unrelated bone marrow or cord blood is better, a retrospective Center for International Blood and Marrow Transplant Research (CIBMTR) study of pediatric acute lymphoblastic leukemia patients undergoing HCT who received 8/8 HLA allele-matched unrelated donor bone marrow was compared with those receiving unrelated cord blood.[16] The analysis showed that the best survival occurred in recipients of 6/6 HLA-matched cord blood; survival after 8/8 HLA-matched unrelated bone marrow was slightly less and was statistically identical to patients receiving 5/6 and 4/6 HLA-matched cord blood units. Based upon these studies, most transplant centers consider matched sibling bone marrow to be the preferred stem cell source/product. If a sibling donor is not available, fully matched unrelated donor bone marrow or PBSCs or HLA matched (4/6 to 6/6) cord blood lead to similar survival. Although adult studies of T-cell depleted unrelated bone marrow or PBSC have shown outcomes similar to non-T-cell depleted approaches, large pediatric trials or retrospective studies comparing T-cell depleted matched or haploidentical bone marrow or PBSCs have not occurred.
Haploidentical HCTEarly HCT studies demonstrated progressively higher percentages of patients experiencing severe GVHD and lower survival as the number of donor/recipient HLA mismatches increased.[29] Studies have further demonstrated that even with very high numbers of donors in unrelated donor registries, patients with rare HLA haplotypes and patients with certain ethnic backgrounds (e.g., Hispanic, African American, Asian-Pacific Islander, etc.) have a low chance of achieving desired levels of HLA matching (7/8 or 8/8 match at the allele level).[7]
In order to allow access to HCT for patients without HLA matched donor options, investigators developed techniques allowing use of siblings, parents, or other relatives who share only a single haplotype of the HLA complex with the patient and are thus “half” matches. The majority of approaches developed to date rely on intense T-cell depletion of the product prior to infusion into the patient. The main challenge associated with this approach is intense immune suppression with delayed immune recovery. This can result in lethal infections,[30] increased risk of EBV-lymphoproliferative disorder, and high rates of relapse.[31] This has generally lead to inferior survival compared with matched HCT and has resulted in the procedure being generally practiced only at larger academic centers with a specific research focus aimed at studying and developing this approach.
Current approaches are rapidly evolving, resulting in improved outcome, with some pediatric groups reporting survival similar to standard approaches.[32,33] Newer techniques of T-cell depletion and add back (i.e., CD3/19 negative selection), have decreased transplant-related mortality.[34] Reduced toxicity regimens have led to improved survival, better supportive care has decreased the chance of morbidity from infection or EBV-lymphoproliferative disorder,[35] and some patient/donor combinations that have specific killer immunoglobulin-like receptor mismatches have shown decreased likelihood of relapse (refer to the Role of killer immunoglobulin-like receptor mismatching in HCT section of this summary for more information). Finally, techniques such as using combinations of granulocyte-colony stimulating factor (G-CSF) primed bone marrow and PBSCs with posttransplant antibody based T-cell depletion [36] or post-HCT cyclophosphamide (chemotherapeutic T-cell depletion) [37] have made these procedures more accessible to centers because expensive and complicated processing necessary for traditional T-cell depletion are not used. Reported survival using many different types of haploidentical approaches varies between 25% to 80% depending upon the technique used and the risk of the patient undergoing the procedure.[31,32,36,37] Whether haploidentical approaches are superior to cord blood or other stem cell sources for a given patient group has not been determined because comparative studies have yet to be performed.[31]
References
- Matthay KK, Villablanca JG, Seeger RC, et al.: Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med 341 (16): 1165-73, 1999. [PUBMED Abstract]
- Woods WG, Neudorf S, Gold S, et al.: A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood 97 (1): 56-62, 2001. [PUBMED Abstract]
- Lawson SE, Harrison G, Richards S, et al.: The UK experience in treating relapsed childhood acute lymphoblastic leukaemia: a report on the medical research council UKALLR1 study. Br J Haematol 108 (3): 531-43, 2000. [PUBMED Abstract]
- Gaynon PS, Harris RE, Altman AJ, et al.: Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children's Oncology Group study CCG-1941. J Clin Oncol 24 (19): 3150-6, 2006. [PUBMED Abstract]
- Schrauder A, von Stackelberg A, Schrappe M, et al.: Allogeneic hematopoietic SCT in children with ALL: current concepts of ongoing prospective SCT trials. Bone Marrow Transplant 41 (Suppl 2): S71-4, 2008. [PUBMED Abstract]
- Pulsipher MA, Peters C, Pui CH: High-risk pediatric acute lymphoblastic leukemia: to transplant or not to transplant? Biol Blood Marrow Transplant 17 (1 Suppl): S137-48, 2011. [PUBMED Abstract]
- Barker JN, Byam CE, Kernan NA, et al.: Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant 16 (11): 1541-8, 2010. [PUBMED Abstract]
- Woolfrey A, Klein JP, Haagenson M, et al.: HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 17 (6): 885-92, 2011. [PUBMED Abstract]
- Shaw PJ, Kan F, Woo Ahn K, et al.: Outcomes of pediatric bone marrow transplantation for leukemia and myelodysplasia using matched sibling, mismatched related, or matched unrelated donors. Blood 116 (19): 4007-15, 2010. [PUBMED Abstract]
- Flomenberg N, Baxter-Lowe LA, Confer D, et al.: Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood 104 (7): 1923-30, 2004. [PUBMED Abstract]
- Petersdorf EW, Kollman C, Hurley CK, et al.: Effect of HLA class II gene disparity on clinical outcome in unrelated donor hematopoietic cell transplantation for chronic myeloid leukemia: the US National Marrow Donor Program Experience. Blood 98 (10): 2922-9, 2001. [PUBMED Abstract]
- Petersdorf EW, Longton GM, Anasetti C, et al.: Definition of HLA-DQ as a transplantation antigen. Proc Natl Acad Sci U S A 93 (26): 15358-63, 1996. [PUBMED Abstract]
- Shaw BE, Gooley TA, Malkki M, et al.: The importance of HLA-DPB1 in unrelated donor hematopoietic cell transplantation. Blood 110 (13): 4560-6, 2007. [PUBMED Abstract]
- Varney MD, Lester S, McCluskey J, et al.: Matching for HLA DPA1 and DPB1 alleles in unrelated bone marrow transplantation. Hum Immunol 60 (6): 532-8, 1999. [PUBMED Abstract]
- Crocchiolo R, Zino E, Vago L, et al.: Nonpermissive HLA-DPB1 disparity is a significant independent risk factor for mortality after unrelated hematopoietic stem cell transplantation. Blood 114 (7): 1437-44, 2009. [PUBMED Abstract]
- Eapen M, Rubinstein P, Zhang MJ, et al.: Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet 369 (9577): 1947-54, 2007. [PUBMED Abstract]
- Eapen M, Klein JP, Sanz GF, et al.: Effect of donor-recipient HLA matching at HLA A, B, C, and DRB1 on outcomes after umbilical-cord blood transplantation for leukaemia and myelodysplastic syndrome: a retrospective analysis. Lancet Oncol 12 (13): 1214-21, 2011. [PUBMED Abstract]
- Barker JN, Scaradavou A, Stevens CE: Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood 115 (9): 1843-9, 2010. [PUBMED Abstract]
- Stevens CE, Carrier C, Carpenter C, et al.: HLA mismatch direction in cord blood transplantation: impact on outcome and implications for cord blood unit selection. Blood 118 (14): 3969-78, 2011. [PUBMED Abstract]
- Barker JN, Rocha V, Scaradavou A: Optimizing unrelated donor cord blood transplantation. Biol Blood Marrow Transplant 15 (1 Suppl): 154-61, 2009. [PUBMED Abstract]
- Wagner JE, Barker JN, DeFor TE, et al.: Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood 100 (5): 1611-8, 2002. [PUBMED Abstract]
- Rubinstein P, Carrier C, Scaradavou A, et al.: Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med 339 (22): 1565-77, 1998. [PUBMED Abstract]
- Barker JN, Weisdorf DJ, DeFor TE, et al.: Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood 105 (3): 1343-7, 2005. [PUBMED Abstract]
- Verneris MR, Brunstein CG, Barker J, et al.: Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood 114 (19): 4293-9, 2009. [PUBMED Abstract]
- MacMillan ML, Weisdorf DJ, Brunstein CG, et al.: Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood 113 (11): 2410-5, 2009. [PUBMED Abstract]
- Brunstein CG, Gutman JA, Weisdorf DJ, et al.: Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood 116 (22): 4693-9, 2010. [PUBMED Abstract]
- Eapen M, Horowitz MM, Klein JP, et al.: Higher mortality after allogeneic peripheral-blood transplantation compared with bone marrow in children and adolescents: the Histocompatibility and Alternate Stem Cell Source Working Committee of the International Bone Marrow Transplant Registry. J Clin Oncol 22 (24): 4872-80, 2004. [PUBMED Abstract]
- Anasetti C, Logan BR, Lee SJ, et al.: Increased incidence of chronic graft-versus-host disease (GVHD) and no survival advantage with filgrastim-mobilized peripheral blood stem cells (PBSC) compared to bone marrow (BM) transplants from unrelated donors: results of Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0201, a phase III, prospective, randomized trial. [Abstract] Blood 118 (21): A-1, 2011.
- Beatty PG, Clift RA, Mickelson EM, et al.: Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med 313 (13): 765-71, 1985. [PUBMED Abstract]
- Aversa F, Tabilio A, Velardi A, et al.: Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med 339 (17): 1186-93, 1998. [PUBMED Abstract]
- Barrett J, Gluckman E, Handgretinger R, et al.: Point-counterpoint: haploidentical family donors versus cord blood transplantation. Biol Blood Marrow Transplant 17 (1 Suppl): S89-93, 2011. [PUBMED Abstract]
- Leung W, Campana D, Yang J, et al.: High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood 118 (2): 223-30, 2011. [PUBMED Abstract]
- González-Vicent M, Molina B, Andión M, et al.: Allogeneic hematopoietic transplantation using haploidentical donor vs. unrelated cord blood donor in pediatric patients: a single-center retrospective study. Eur J Haematol 87 (1): 46-53, 2011. [PUBMED Abstract]
- Handgretinger R, Chen X, Pfeiffer M, et al.: Feasibility and outcome of reduced-intensity conditioning in haploidentical transplantation. Ann N Y Acad Sci 1106: 279-89, 2007. [PUBMED Abstract]
- Leen AM, Christin A, Myers GD, et al.: Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood 114 (19): 4283-92, 2009. [PUBMED Abstract]
- Huang XJ, Liu DH, Liu KY, et al.: Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 38 (4): 291-7, 2006. [PUBMED Abstract]
- Luznik L, Fuchs EJ: High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res 47 (1-3): 65-77, 2010. [PUBMED Abstract]


 Back to Top
Back to Top