Principles of HCT Preparative Regimens
In the days just prior to infusion of the stem cell product (bone marrow, peripheral blood stem cell, or cord blood), hematopoietic cell transplantation (HCT) recipients receive chemotherapy/immunotherapy sometimes combined with radiation therapy. This is called a preparative regimen and the original intent of this treatment was to:
- Create bone marrow space in the recipient for the donor cells to engraft.
- Suppress the immune system or eliminate the recipient T-cells to minimize risks of rejection.
- Intensely treat cancer (if present) with mega-dose therapy of active agents with the intent to overcome therapy resistance.
With the recognition that donor T-cells can facilitate engraftment and kill tumors through graft-versus-leukemia effects (obviating the need to create bone marrow space and intensely treat cancer), reduced-intensity or minimal-intensity HCT approaches focusing on immune suppression rather than myeloablation have been developed. The resultant lower toxicity associated with these regimens has led to lower rates of transplant-related mortality and an expansion eligibility for allogeneic HCT to older individuals and younger patients with pre-HCT comorbidities that put them at risk for severe toxicity after standard HCT approaches.[1] The many preparative regimens available now vary tremendously in the amount of immunosuppression and myelosuppression that they cause, with the lowest intensity regimens relying heavily on a strong graft-versus-tumor effect.
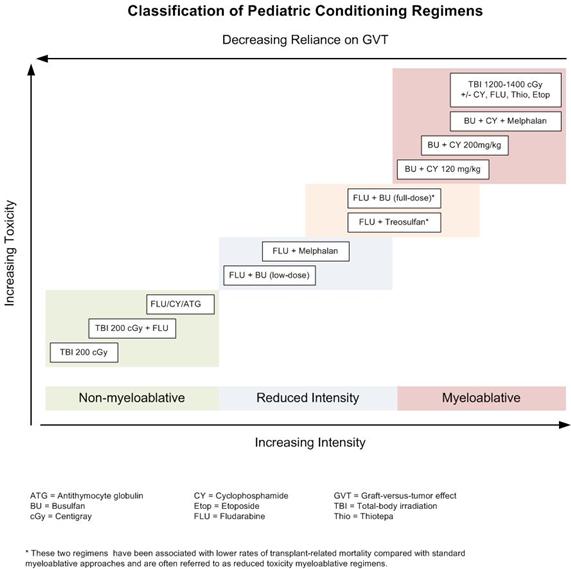
Although these regimens represent a spectrum of varying degrees of myelosuppression and immune suppression, they have been categorized clinically in the following three major categories:[2]
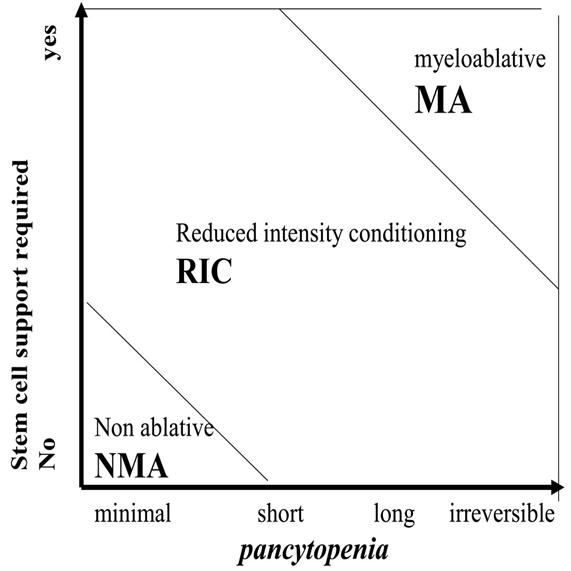
- Myeloablative: Intense approaches that cause irreversible pancytopenia requiring stem cell rescue for restoration of hematopoiesis.
- Nonmyeloablative: Regimens that cause minimal cytopenias and do not require stem cell support.
- Reduced-intensity conditioning: Regimens that are of intermediate intensity and do not meet the definitions of nonmyeloablative or myeloablative regimens.
The use of reduced-intensity conditioning and nonmyeloablative regimens is well-established in older adults who cannot tolerate more intense myeloablative approaches,[3-5] but only a handful of younger patients with malignancies have been studied using these approaches.[6-10] A large Pediatric Blood and Marrow Transplant Consortium study identified patients at high risk for transplant-related mortality with myeloablative regimens (e.g., history of previous myeloablative transplant, severe organ system dysfunction, or active invasive fungal infection) and successfully treated them with a reduced-intensity regimen.[11] Transplant-related mortality was low in this high-risk group, and long-term survival occurred in most patients with minimal or no detectable disease present at the time of transplantation. Because the risks of relapse are higher with these approaches, their use in pediatric cancer is currently limited to patients ineligible for myeloablative regimens.
Establishing Donor ChimerismIntense myeloablative approaches almost invariably result in rapid establishment of hematopoiesis derived completely from donor cells upon count recovery within weeks of the transplant. The introduction of reduced-intensity conditioning and nonmyeloablative approaches into HCT practice has resulted in a slower pace of transition to donor hematopoiesis (gradually increasing from partial to full donor hematopoiesis over months) that is sometimes only partial. DNA-based techniques have been established to differentiate donor and recipient hematopoiesis, applying the word chimerism (from the Greek chimera, a mythical animal with parts taken from various animals) to describe whether all or part of hematopoiesis after HCT is from the donor or recipient.
There are several implications to the pace and extent of donor-chimerism eventually achieved by an HCT recipient. For patients receiving reduced-intensity conditioning or nonmyeloablative regimens, rapid progression to full donor chimerism is associated with less relapse, but more graft-versus-host disease (GVHD).[12] The delayed pace of obtaining full-donor chimerism after these regimens has led to late-onset acute GVHD, occurring as much as 6 months to 7 months after HCT (generally within 100 days after myeloablative approaches).[13] A portion of patients achieve stable mixed chimerism of both donor and recipient. Mixed chimerism is associated with more relapse after HCT for malignancies and less GVHD; however, this condition is often advantageous for nonmalignant HCT, where usually only a percentage of normal hematopoiesis is needed to correct the underlying disorder and GVHD is not beneficial.[14] Finally, serially measured decreasing donor chimerism, especially T-cell specific chimerism, has been associated with increased risk of rejection.[15]
Because of the implications of persistent recipient chimerism, most transplant programs test for chimerism shortly after engraftment and continue testing regularly until stable full donor hematopoiesis has been achieved. Investigators have defined two approaches to treat the increased risks of relapse and rejection associated with increasing recipient chimerism: rapid withdrawal of immune suppression and donor lymphocyte infusions (DLI). These approaches are frequently used to address this issue, and have been shown in some cases to decrease relapse risk and stop rejection.[16,17] Timing of tapers of immune suppression and doses and approaches to the administration of DLI to increase or stabilize donor chimerism vary tremendously between transplant regimens and institutions.
References
- Deeg HJ, Sandmaier BM: Who is fit for allogeneic transplantation? Blood 116 (23): 4762-70, 2010. [PUBMED Abstract]
- Bacigalupo A, Ballen K, Rizzo D, et al.: Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 15 (12): 1628-33, 2009. [PUBMED Abstract]
- Giralt S, Estey E, Albitar M, et al.: Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood 89 (12): 4531-6, 1997. [PUBMED Abstract]
- Slavin S, Nagler A, Naparstek E, et al.: Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 91 (3): 756-63, 1998. [PUBMED Abstract]
- Storb R, Yu C, Sandmaier BM, et al.: Mixed hematopoietic chimerism after marrow allografts. Transplantation in the ambulatory care setting. Ann N Y Acad Sci 872: 372-5; discussion 375-6, 1999. [PUBMED Abstract]
- Bradley MB, Satwani P, Baldinger L, et al.: Reduced intensity allogeneic umbilical cord blood transplantation in children and adolescent recipients with malignant and non-malignant diseases. Bone Marrow Transplant 40 (7): 621-31, 2007. [PUBMED Abstract]
- Del Toro G, Satwani P, Harrison L, et al.: A pilot study of reduced intensity conditioning and allogeneic stem cell transplantation from unrelated cord blood and matched family donors in children and adolescent recipients. Bone Marrow Transplant 33 (6): 613-22, 2004. [PUBMED Abstract]
- Gómez-Almaguer D, Ruiz-Argüelles GJ, Tarín-Arzaga Ldel C, et al.: Reduced-intensity stem cell transplantation in children and adolescents: the Mexican experience. Biol Blood Marrow Transplant 9 (3): 157-61, 2003. [PUBMED Abstract]
- Pulsipher MA, Woolfrey A: Nonmyeloablative transplantation in children. Current status and future prospects. Hematol Oncol Clin North Am 15 (5): 809-34, vii-viii, 2001. [PUBMED Abstract]
- Roman E, Cooney E, Harrison L, et al.: Preliminary results of the safety of immunotherapy with gemtuzumab ozogamicin following reduced intensity allogeneic stem cell transplant in children with CD33+ acute myeloid leukemia. Clin Cancer Res 11 (19 Pt 2): 7164s-7170s, 2005. [PUBMED Abstract]
- Pulsipher MA, Boucher KM, Wall D, et al.: Reduced-intensity allogeneic transplantation in pediatric patients ineligible for myeloablative therapy: results of the Pediatric Blood and Marrow Transplant Consortium Study ONC0313. Blood 114 (7): 1429-36, 2009. [PUBMED Abstract]
- Baron F, Baker JE, Storb R, et al.: Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood 104 (8): 2254-62, 2004. [PUBMED Abstract]
- Vigorito AC, Campregher PV, Storer BE, et al.: Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood 114 (3): 702-8, 2009. [PUBMED Abstract]
- Marsh RA, Vaughn G, Kim MO, et al.: Reduced-intensity conditioning significantly improves survival of patients with hemophagocytic lymphohistiocytosis undergoing allogeneic hematopoietic cell transplantation. Blood 116 (26): 5824-31, 2010. [PUBMED Abstract]
- McSweeney PA, Niederwieser D, Shizuru JA, et al.: Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 97 (11): 3390-400, 2001. [PUBMED Abstract]
- Bader P, Kreyenberg H, Hoelle W, et al.: Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? J Clin Oncol 22 (9): 1696-705, 2004. [PUBMED Abstract]
- Horn B, Soni S, Khan S, et al.: Feasibility study of preemptive withdrawal of immunosuppression based on chimerism testing in children undergoing myeloablative allogeneic transplantation for hematologic malignancies. Bone Marrow Transplant 43 (6): 469-76, 2009. [PUBMED Abstract]


 Back to Top
Back to Top