|
No instant look-up matches. Search within full reference content by clicking the "SEARCH" button or pressing enter. |
Latest Updates in Medscape Today
Academic Partners
|
|
Advisory Board
|
|
-
 Best Practices: Treating Hypothermic Patients
Best Practices: Treating Hypothermic Patients
-
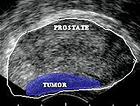 Prostate Cancer: Definitive Diagnosis and Staging
Prostate Cancer: Definitive Diagnosis and Staging
-
 Autoimmune Disorders: Keys to Early Diagnosis and Treatment
Autoimmune Disorders: Keys to Early Diagnosis and Treatment
-
 Can't-Miss Complications of Chronic Alcohol Abuse
Can't-Miss Complications of Chronic Alcohol Abuse
-
 Cutaneous Clues to Diagnosing Metastatic Cancer
Cutaneous Clues to Diagnosing Metastatic Cancer
-
 Diagnosing Dermatoses in Pregnant Patients
Diagnosing Dermatoses in Pregnant Patients
-
 Best Practices: Tube Thoracostomy Insertion
Best Practices: Tube Thoracostomy Insertion
-
 Can't-Miss Testicular Pathology
Can't-Miss Testicular Pathology
-
 Rare and Unusual Complications of Twin Pregnancies
Rare and Unusual Complications of Twin Pregnancies
-
 Clues on the Skin: Acute Poisonings
Clues on the Skin: Acute Poisonings
Medscape Reference is the most authoritative and accessible point-of-care medical reference for physicians and health care professionals. All content is available free of charge, both online and via mobile devices.
Medscape Reference articles represent the expertise and practical knowledge of top physicians and pharmacists from leading academic medical centers in the US and worldwide. In addition, our rigorous literature survey process allows us to rapidly integrate new practice-changing information into the relevant topics by systematically reviewing the major medical and pharmacy journals, news announcements, and important practice guidelines.
Medscape Reference provides comprehensive coverage across more than 30 medical specialties and is composed of the following areas:
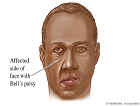
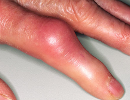
Diseases and ConditionsMore than 6,300 evidence-based and physician-reviewed disease and condition articles are organized to rapidly and comprehensively answer clinical questions, as well as to provide in-depth information in support of diagnosis, treatment, and other clinical decision-making. Topics are richly illustrated with more than 30,000 clinical photos and radiographic images.
ProceduresMore than 650 clinical procedure articles provide clear, step-by-step instructions, and include instructional videos and images to allow clinicians to master the newest techniques or to improve their skills in procedures they have previously performed.
DrugsMore than 2,100 monographs, including prescription and over-the-counter drugs, plus over 5,000 corresponding brand-name drugs, herbals, and supplements, are provided. Drug images and pricing are also included. In addition, a Drug Interaction Checker provides rapid access to tens of thousands of interactions between brand and generic drugs, over-the-counter drugs, and herbals and supplements.
AnatomyMore than 70 anatomy articles feature clinical images and diagrams of the major human body systems and organs. The articles assist with the understanding of the anatomy involved in treating specific conditions and performing procedures. They can also facilitate physician-patient discussions.
Special FeaturesBest Evidence
This email newsletter features current and clinically relevant journal citations that are selected via our rigorous literature survey review process. These same citations are incorporated into the relevant Medscape Reference articles. This newsletter serves as a quick and easily accessible way to stay up to date with clinically relevant journal articles that impact clinical practice.
Case StudiesThese challenging and classic case presentations provide a quick way for practicing physicians to sharpen their diagnostic and patient-management skills. Each case starts with how the patient presented in the clinic and then using a question and answer format, progresses through diagnosis and on to treatment and includes follow up information about outcome for the patient.
Image collectionsImage collections are a visually-engaging presentation of both common and uncommon diseases, case presentations, and current controversies in medicine. They are presented in an image rich, slideshow format and are designed to challenge and expand physicians' knowledge of clinically important topics.
Medscape is the leading online destination for healthcare professionals for clinical information. In addition to clinical reference tools, Medscape also offers:
Medscape News Learn moreMedscape Education Learn more