National Comprehensive Cancer Network
NCCN Research & Business Resources
NCCN Oncology Outcomes Database
The NCCN Oncology Outcomes Database contains expansive data on drug/biologic and diagnostics utilization and trends; specific indications and sequencing; toxicity and reasons for discontinuation; complications and
medical events; progression free and overall survival; comparative effectiveness and resource consumption; and payor-specific data.
A unique database by any measure:
- More than 300 data elements tracking the continuum of care longitudinally
- All clinical interventions collected, including specific regimens, lines of therapy, and oral agents
- Clinical trial quality data
- HIPAA compliant
- Since 1997, the NCCN Database has developed, enhanced, and expanded the data available for Breast, Colon/Rectal, Lung, and Ovarian Cancers and Non-Hodgkin’s Lymphomas*
For more information on the NCCN Database or other inquiries, please contact Christine MacCracken, MSHEd, BSN, Senior Director, Business Insights.
The NCCN Oncology Outcomes Database is a network-based data collection, reporting, and analytic system that describes the patterns and outcomes of care delivered in the management of patients with cancer. The concept for the NCCN Database was established in 1996, and the operation of the first database in breast cancer was initiated in July of 1997. With the NCCN Oncology Outcomes Database, NCCN seeks to implement the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) through performance measurement. The objectives for data collection and reporting are to:
- Describe the patterns of care within NCCN Member Institutions
- Measure concordance of practice in NCCN Member Institutions with NCCN Guidelines recommendations
- Evaluate the outcomes of such practice patterns
- Deliver benchmarked data to participating NCCN Member Institutions, practicing physicians, and NCCN Guidelines Panels to facilitate the continuous improvement of the quality of cancer care
- Identify the most efficient and cost-effective strategies for the management of common oncologic conditions
- Offer benchmarking capabilities to NCCN Member Institutions comparing network data, institutional data, and, eventually, community data
- Create a major research resource and repository of data for clinical and health service researchers to access and derive hypothesis-generated analyses
Bibliography: NCCN Oncology Outcomes Database Manuscripts
Bibliography: NCCN Oncology Outcomes Database Abstracts
For more information, please contact Christine MacCracken, MSHEd, BSN, Senior Director, Business Insights.
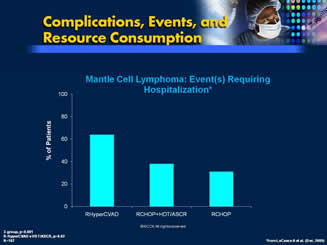
Sample Data:
 |
 |
 |
 |
 |
 |
 |
 |
 |
| Quick Links |
|
|
About NCCN| NCCN Member Institutions| Patient Resources| NCCN Foundation| Privacy Policy| Legal Notices| Contact Us
275 Commerce Drive, Suite 300, Fort Washington, PA 19034 • 215.690.0300 • Fax: 215.690.0260
Copyright © 2013 National Comprehensive Cancer Network, All Rights Reserved
