FYI from the NHLBI Index
September 2002: Vol. 3, Issue 2
Feature Articles
Estrogen Plus Progestin Trial Stopped Due to Increased Risk, Lack of Overall Benefit
NIH Seeks Applicants for the Director's Council of Public Representatives
Estrogen Plus Progestin Trial Stopped Due to Increased Risk, Lack of Overall Benefit
On July 9, 2002, the NHLBI stopped the Women's Health Initiative (WHI) estrogen plus progestin trial because researchers found that the risks of long-term estrogen plus progestin therapy outweigh its protective benefits. Specifically, the researchers found increased risks of heart attacks, stroke, invasive breast cancer, and blood clots in study participants taking estrogen plus progestin compared with women taking placebo pills. There were noteworthy benefits of estrogen plus progestin, including fewer cases of colon cancer and hip fractures, but on balance, the harm was greater than the benefit.
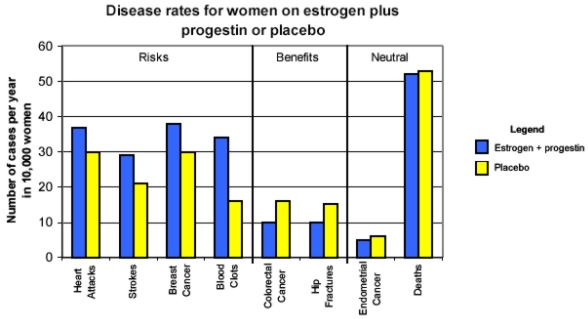
Graph data in table format.
According to the trial findings, the increased risk of breast cancer, cardiovascular disease, or stroke for each woman taking the estrogen plus progestin therapy was actually very small. For example, each woman who took the estrogen plus progestin therapy had an increased risk of breast cancer of less than a tenth of 1 percent per year. However, the small increases in risk apply to the entire population of women on the therapy and over several years, so the potential public health impact is considerable.
Choosing whether to use postmenopausal hormone therapy is one of the most important health decisions women face as they age, and the WHI study provides important new information. Based on the findings, the WHI investigators' recommendations for estrogen plus progestin use are:
- The therapy should not be continued or started to prevent heart disease. Women should consult their doctor about other methods of prevention, such as lifestyle changes and cholesterol- and blood pressure-lowering drugs.
- For osteoporosis prevention, women should consult their doctor and weigh the benefits against their personal risks for heart attack, stroke, blood clots, and breast cancer. Alternative treatments also are available to prevent osteoporosis and fractures.
- Women should keep up with their regular schedule of mammograms and breast self-examinations.
- Although short-term use was not studied, women taking the therapy for relief of menopausal symptoms may find that the benefits justify the risks. Women should talk with their doctor about their personal risks and benefits.
For more information on the estrogen plus progestin trial results and the WHI, visit www.nhlbi.nih.gov/whi/hrtupd.
NIH Seeks Applicants for the Director's Council of Public Representatives
 Looking
to get more involved with the NIH? The NIH Director's Council of Public Representatives
(COPR -- pronounced "copper") currently is seeking applicants. The COPR serves
as an important forum for information exchange between the public and the NIH.
It consists of up to 21 individuals who are selected from among the many diverse
communities that benefit from, and have an interest in, NIH research, programs,
and activities. They are patients, family members of patients, health care professionals,
members of patient or not-for-profit groups, scientists, health and science
educators, and they could include you. View the COPR
Application Package online for details on how to apply. The deadline for
applications is September 16, 2002, with final selections to be announced in
spring 2003.
Looking
to get more involved with the NIH? The NIH Director's Council of Public Representatives
(COPR -- pronounced "copper") currently is seeking applicants. The COPR serves
as an important forum for information exchange between the public and the NIH.
It consists of up to 21 individuals who are selected from among the many diverse
communities that benefit from, and have an interest in, NIH research, programs,
and activities. They are patients, family members of patients, health care professionals,
members of patient or not-for-profit groups, scientists, health and science
educators, and they could include you. View the COPR
Application Package online for details on how to apply. The deadline for
applications is September 16, 2002, with final selections to be announced in
spring 2003.
Please send us your feedback, comments, and questions by using the appropriate link on the page, Contact the NHLBI.
Note to users of screen readers and other assistive technologies: please report your problems here.
All Issues | FYI Index | NHLBI Express














 Twitter
Twitter
 Facebook
Facebook YouTube
YouTube