Food
Guidance for Industry: The Seafood List - FDA's Guide to Acceptable Market Names for Seafood Sold in Interstate Commerce
Contains Nonbinding Recommendations
Additional copies are available from:
Office of Food Safety
Division of Seafood Safety HFS-325
Center for Food Safety and Applied Nutrition
Food and Drug Administration
5100 Paint Branch Parkway
College Park, MD 20740
(Tel) 301-436-2300 (Updated phone: 240-402-2300)
http://www.fda.gov/FoodGuidances
You may submit written comments regarding this guidance at any time. Submit written comments on the guidance to the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852. All comments should be identified with the title of the guidance document.
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Food Safety and Applied Nutrition
Issued: September 1993
Revised: January 2009
November 2010
Contains Nonbinding Recommendations
Table of Contents
- Introduction
- Background
- Discussion
- The Seafood List
- Understanding and Using The Seafood List
- Principles for Determining Acceptable Market Names
- References
Contains Nonbinding Recommendations
Guidance for Industry[1]
The Seafood List
FDA's Guide to Acceptable Market Names for
Seafood Sold in Interstate Commerce
This guidance represents the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the FDA staff responsible for implementing this guidance. If you cannot identify the appropriate FDA staff, call the number listed on the title page of this guidance.
I. Introduction
This guidance is intended to provide guidance to industry about what FDA considers to be acceptable market names for seafood sold in interstate commerce and to assist manufacturers in labeling seafood products. This guidance defines the different categories of names found in The Seafood List and outlines principles that can be used to label seafood species sold in the United States (U.S.) with an appropriate, nonmisleading statement of identity.
FDA's guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe the Agency's current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required.
II. Background
Through the years, the Federal Government has worked to provide consistent and scientifically sound recommendations to industry and consumers about acceptable market names for seafood sold in interstate commerce. This advice was consolidated in 1988 when The Fish List was first published by FDA in cooperation with the National Marine Fisheries Service to provide a source of names that would facilitate consistency and order in the U.S. market place and reduce confusion among consumers. Although The Fish List had significant success in achieving its goal, its usefulness was limited because it did not include invertebrate species. In 1993, The Fish List was revised to include the acceptable market names for domestic and imported invertebrate species sold in interstate commerce, and renamed The Seafood List. The Seafood List provides information to assist manufacturers in properly labeling seafood and to reflect the acceptable market names of new species introduced into the U.S. marketplace.
III. Discussion
The use of acceptable market names is essential in the identification of seafood because of the exceptional number and variety of species represented by this unique category of foods. The unparalleled diversity in this category of similar foods means that very few species have just one nationally recognized, common or usual name that allows consumers to unambiguously identify a species in the marketplace. Typically, even the most popular and widely consumed species have acceptable market names that are shared with other species. For example, "salmon," "bass," "tuna," "cod," "halibut," and "snapper" are names commonly used to identify particular species of fish, but these are also names that are sometimes used to represent a group of finfish species. When used as the market name, the group name may properly encompass and adequately identify for consumers any member of the group, but it does not provide enough information for a consumer to identify the specific species, if a consumer desires that level of specificity. The "scientific common name" generally provides that level of specificity and usually is also an acceptable market name.
IV. The Seafood List
When determining how to appropriately label seafood, one should either check The Seafood List, or type in the species name using the search box below to identify acceptable market names.
V. Understanding and Using The Seafood List
Species that are included on The Seafood List are those that FDA has determined are sold in interstate commerce or are likely to be sold in interstate commerce and are not prohibited by law from sale in interstate commerce.
The column headings in The Seafood List identify four types of names for each species, i.e., market name, scientific common name, scientific name. Additionally, vernacular names are provided in The Seafood List only to assist with cross-referencing to an acceptable market name.
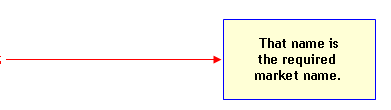
- Acceptable Market Name: An acceptable market name is a name that FDA recognizes as a suitable "statement of identity" (21 CFR 101.3) in the labeling of a species. An acceptable market name fairly represents the identity of the species to U.S. consumers because it is not confusingly similar to the name of another species and because it is not otherwise misleading. An acceptable market name may be: (1) a "common or usual name" established by either a history of common usage in the U.S. or by regulation; (2) the "scientific common name"; or (3) more rarely, a name specifically coined as the market name for a species, e.g., "basa" is the market name coined for Pangasius bocourti). The market names of the species that are required by regulation are marked with an asterisk (*) in The Seafood List. The specific regulation is noted to provide additional information.
- Scientific Common Name: The scientific common name is the English version of the name established and commonly used by ichthyologists and other fishery experts to describe a specific species, and is distinct from the scientific name. In some cases, the market name and the scientific common name are the same. Almost all species have been assigned a unique scientific common name and their use as market names has the advantage of limiting confusion about species identity in the marketplace. For this reason, FDA generally recommends use of the scientific common name as the market name unless a common or usual name has been established by regulation or law. Scientific common names used for species in the entire genera are in parenthesis. There are some scientific common names that are prohibited by law and cannot be used as market names. The scientific common names of the species that are prohibited by a law are marked with a dagger (†) in The Seafood List. The specific law is noted to provide additional information.
- Scientific Name: The scientific name is the Latin name for the genus and species of a fish, established by fisheries taxonomists. These names are included in the list to permit exact species identification and are not, by themselves, acceptable market names, although they may be used to supplement an acceptable market name in labeling. We have attempted to list valid scientific names. However, some synonyms are listed because of name changes based on advancing knowledge and views of taxonomic specialists.
- Vernacular Name: A vernacular name is a commonly recognized local or regional name for a species. In many cases, the same species may be recognized by a different name in another locale or region. Vernacular names generally are not acceptable market names and their use as such may result in misbranding. Vernacular names are provided in The Seafood List only to assist with cross-referencing to an acceptable market name.
VI. Principles for Determining Acceptable Market Names
FDA generally evaluates whether a name is an acceptable market name based on the following principles
Principle 1:
A common or usual name required by regulation or law is the required market name of the food (i.e., the "statement of identity" (21 CFR 101.3)).
Common or usual names currently required by regulation or law are
- Pacific whiting (21 CFR 102.46),
- Bonito (21 CFR 102.47),
- Crabmeat (21 CFR 102.50),
- Greenland turbot (21 CFR 102.57),
- Canned oysters (21 CFR 161.145),
- Canned Pacific salmon (21 CFR 161.170),
- Canned tuna (21 CFR 161.190), and
- Catfish (Federal Food, Drug, and Cosmetic Act (FD&C Act); Sec. 403(t) (21 U.S.C. 343(t)).
In The Seafood List the market names of the species that are required by regulation are marked with an asterisk (*). The scientific common names that are prohibited by law and cannot be used as market names are marked with an dagger (†); e.g. Walking Clarias Fish is an acceptable market name; "Walking Catfish" (†) is not an acceptable market name.
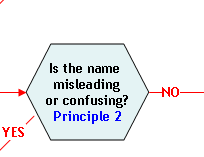
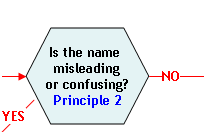
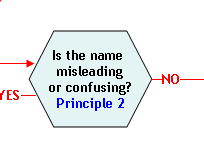
Principle 2:
A name that is false or misleading is not an acceptable market name.
A food is deemed to be misbranded under section 403(a)(1) of the FD&C Act (21 U.S.C. 343(a)(1)) if "...its labeling is false or misleading in any particular…" Some examples of how a market name may be false or misleading to a consumer are:
- The name is not the name required by law or regulation.
- The name is the same as the name of another species or is confusingly similar to the name of another species and it is not reasonably encompassed within a group of species so named.
- The name implies a unique geographical origin that is misleading.
- The name is a fanciful or coined name that inaccurately characterizes the
quality, value, or other feature of the species.
The use of a false or misleading name may prevent correct species identification and thereby affect the ability of processors and consumers to make accurate assessments of the potential safety hazards associated with seafood. Hazards such as allergenic proteins and scombrotoxin formation are associated with some species but not others, presenting potential food safety risks if the food is not accurately labeled. Misbranding may also result in economic fraud, because of the difference in the market value of different but similar species of fish. Geographical designations used as part of a market name should truthfully represent the geographical origin of the species (21 CFR 101.18(c)), or otherwise conform to the provisions of 21 CFR 101.18(c)(4). Geographical designations are sometimes part of a species' common or usual name (e.g., Atlantic salmon). FDA recognizes that these descriptors and those used in some scientific common names can be truthful and meaningful parts of a food's statement of identity. Other uses of a geographical descriptor, for example as part of a coined or fanciful name where the descriptor does not accurately describe the geographical extent of the source of the species, may be misleading and are not recommended. The global spread of aquaculture may further confound the use of place of origin labeling, because a species with natural origins in one region of the world may be aquacultured in another region (e.g., Ictalurus puntatus). Thus, acceptable references in a market name to source waters or place of geographical origin should be used only to describe a species from an exclusive geographical source or fishery, consistent with the requirements of 21 CFR101.18(c).
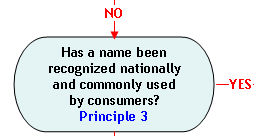
Principle 3:
A name that has been recognized nationally in the U.S. and commonly used by consumers to identify a species may be an acceptable market name.
A name that has been nationally recognized in the U.S. and commonly used by consumers to identify a species is a common or usual name of a food (21 CFR 102.5(a)) that may be an acceptable market name. Some of the most frequently consumed species in The Seafood List have acceptable market names that are widely recognized by U.S. consumers as referring to a group of similar, related species. For example, the names "tuna," "salmon," and "grouper" can each be used to refer to a variety of species of finfish. Individual species are at times differentiated by acceptable market names that are recognized nationally and commonly used by consumers. Yellowfin tuna, sockeye salmon, and coho salmon are examples of differentiated marketed names. The use of a single unique name for any food is the optimum situation for an orderly market. FDA recognizes, however, that there are instances where more than one acceptable market name for a species is recognized in the marketplace. Moreover, these names may have been used interchangeably in the U.S. without causing confusion or misrepresentation of the true character and identity of the species involved (e.g., "monkfish" and "goosefish" are acceptable market names that may be used interchangeably for various Lophius spp. Conversely, as noted above, several different species may share an acceptable market name without causing apparent market conflict, i.e., the market name "grouper" may be used for several Epinephelus spp. To the extent possible, market names should provide a clear distinction between species that have different qualities and value to consumers (e.g., "pollock" and "cod" are distinct names for distinct species and consumers generally associate higher quality and value with "cod"). FDA also recommends that the market name allow for the selection by consumers from among species with similar names. Market names that express species qualities can be achieved by using the scientific common name, as opposed to the market name (e.g., "snapper" and "red snapper" for which the species Lutjanus campechanus, i.e., "red snapper," is more highly valued than other Lutjanus spp. that may be called "snapper." (FDA Compliance Policy Guide Sec. 540.475 Snapper - Labeling (CPG 7108.21).
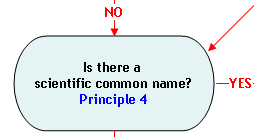
Principle 4:
The scientific common name is generally an acceptable market name.
The name commonly used to identify the same fish may vary from region to region. A vernacular name (local or regional name) for a species, even though well established in that area, may not be an acceptable market name for use in interstate commerce. In the broader marketplace, a vernacular name may be the same as, or confusingly similar to the name used to identify a different species in another region. Market name conflicts can be avoided by using species-specific scientific common names that are assigned by taxonomists. Because of their specificity, scientific common names allow consumers to more readily differentiate between similar species. FDA generally regards scientific common names as appropriate market names, provided they are not misleading or confusing (Principle 2). FDA generally recommends use of the scientific common name as the market name unless a common or usual name has been established by regulation or law. There are some scientific common names that are prohibited by law and cannot be used as market names. The scientific common names of the species that are prohibited by a law are marked with an dagger (†) in The Seafood List. The specific law is noted to provide additional information.
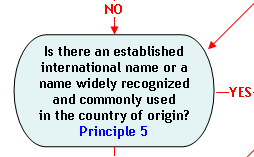
Principle 5:
An established international name (e.g., established by the Food and Agriculture Organization of the United Nations (FAO)) or a name that is widely recognized and commonly used in the country of origin may be an acceptable market name.
When a species is first introduced into the U.S. market, whether as a new import or a new hybrid, a market name normally is not available. In these instances, an internationally recognized name, such as one recognized by FAO may be an acceptable market name. For example, when a particular species of crab was first introduced in the U.S. market, Principles l and 3 could not be used to develop an acceptable market name. The FAO-recognized name "purple stone crab" was identified as a common name and FDA concluded that this name would be an acceptable market name. Alternatively, a name that is widely recognized and commonly used by consumers in the country of origin may be an acceptable market name. However, neither an internationally recognized name nor the name most commonly used in other countries will be an acceptable market name if it is misleading or confusing (Principle 2).
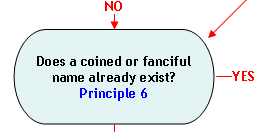
Principle 6:
A coined name may be an acceptable market name.
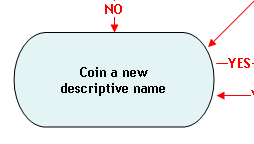
When other naming conventions are not available, a coined or fanciful name may be an acceptable market name, provided the name is not misleading or confusing (Principle 2). Coined names are useful when a species has an otherwise unappealing scientific common name (e.g., the coined name "orange roughy" is more appealing than the scientific common name "slime head"), or when the use of a market or scientific common name is prohibited by regulation or law. For example, the market name "basa catfish" was used for Pangasius bocourti prior to the passage of section 403(t) of the FD&C Act (21 U.S.C. 343(t)). Under section 403(t) of the Act, a food is deemed misbranded if it purports to be or is represented as catfish, unless it is fish classified within the family Ictaluridae. Thus, the market name "basa" was coined for Pangansius bocourti. If an acceptable coined or fanciful name does not already exist, one may coin a new descriptive name provided that it is not misleading or confusing (Principle 2).
Flowchart for Selecting an Acceptable Market Name
 |
 |
|
 |
||
 |
 |
|
 |
 |
|
 |
 |
|
 |
 |
|
 |
 |
|
VII. References
FDA has verified the cited Web addresses, but is not responsible for subsequent changes to them after this document issues.
- Food and Drug Administration, "Fish and Fishery Products Hazards and Controls Guidance, Third Edition," Chapter 3. 2001.
- Joseph S. Nelson, Edwin J. Crossman, Hector Espinosa-Perez, Lloyd T. Findley, Carter R. Gilbert, Robert N. Lea, and James D. Williams, "Common and Scientific Names of Fishes From the United States, Canada and Mexico," American Fisheries Society, Sixth edition, 2004.
- Donna Turgeon et al., "Common and Scientific Names of Aquatic Invertebrates from the United States and Canada: Mollusks," American Fisheries Society, Second Edition 1998.
- Williams et al., "Common and Scientific Names of Aquatic Invertebrates from the United States and Canada: Decapod Crustaceans," Publication 17. American Fisheries Society, 1989.
- R. Robins et al., "World Fishes Important to North Americans Exclusive of Species from the Continental Waters of the United States and Canada," American Fisheries Society, Publication 21; 1991.
- Willibald Krane, "Five-Language Dictionary of Fish, Crustaceans and Molluscs," Van Nostrand Reinhold, 1986.
- Organization for Economic Co-operation and Development, "Multilingual Dictionary of Fish and Fish Products," Fourth Edition, Fishing News Books, New York 1995.
- Austin Williams, "Lobsters of the World, an Illustrated Guide, Lobsters of the World in U.S. Trade," Osprey Books, 1988.
- J. McClane, Holt, Rinehart and Winston, "The Encyclopedia of Fish Cookery," First Edition Canada 1977.
- Integrated Taxonomic Information System: ITIS on-line database (accessed 12/03/2009).
- FishBase
 : A Global Information System on Fishes, Froese, R., Bailly and D. Pauly, Editors (accessed 12/03/2009).
: A Global Information System on Fishes, Froese, R., Bailly and D. Pauly, Editors (accessed 12/03/2009).
(1) This guidance has been prepared by the Division of Seafood Safety in the Center for Food Safety and Applied Nutrition at the U.S. Food and Drug Administration.
The FDA Seafood List - Recent Updates and Additions