For Health Care Professionals/Providers
Health eCard
 For Patient Reminder/Recall
For Patient Reminder/Recall
Preteen and teen vaccines are on our radar. Are they on yours?
Reminder Systems and Strategies for Increasing Vaccination Rates
The resources below can be used to assist doctors, nurses and other health professionals in speaking with patients and their parents about adolescent vaccines.
Provider Factsheet [color - 308 KB, 4 pages]*
Factsheet about adolescent vaccines developed specifically for the doctors, nurses and other health care professionals. Also available in black & white [214 KB, 4 pages]
Vaccine Recommendations & Administration

- Adolescent immunization schedule (7-18 years old)
- Screening Questionnaire Teen Immunization [108 KB, 2 pages]
Tool for determining which vaccines may be needed for your 9-18 year old patients - International travel
Use the Yellow Book to determine vaccines and medications necessary to prevent illness when traveling to other countries. Or, consult the destination map to identify vaccines needed before your patients travel.
Printer-friendly version of chart below [63 KB, 1 page]
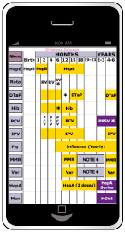
| Tdap | HPV | MCV4 | Flu |
|---|---|---|---|
Tetanus & diphtheria toxoids & acellular pertussis vaccine (Tdap) SUMMARY:
|
Human papillomavirus vaccine (HPV) SUMMARY:
|
Meningococcal conjugate vaccine, quadrivalent (MCV4) SUMMARY:
|
Influenza vaccine (seasonal) SUMMARY:
|
Vaccines For Children (VFC) Program
The Vaccines for Children (VFC) program provides vaccines at no cost to professionals who serve eligible children. Children younger than 19 years of age are eligible for VFC vaccines if they are Medicaid-eligible, American Indian or Alaska Native or have no health insurance. Children who have health insurance that does not cover vaccination can receive VFC vaccines through Federally Qualified Health Centers or Rural Health Centers. VFC vaccines cannot be denied to an eligible child if a family can’t afford the administration fee. Go to the VFC web site for more information about participating in VFC.
Provider Education
- Adolescent Immunizations: A Back-to-School Checklist 29:32 minutes
CDC and Medscape Training
CME/CE activity for physicians, nurses, and pharmacists who recommend or provide vaccinations to preteens and teens. Goals are to improve knowledge of Advisory Committee on Immunization Practices (ACIP) recommendations for vaccination of adolescents and to increase application of the recommended vaccination schedule. July 2012 - HPV Vaccine: A Shot of Cancer Prevention 18:45 minutes
CDC and Medscape Training
CME/CE activity for physicians, nurses, and pharmacists who recommend or provide vaccinations to preteens and teens. The goals of this activity are to increase clinician recognition of the burden of HPV-related disease and to increase understanding of Advisory Committee on Immunization Practices (ACIP) recommendations for HPV disease prevention through vaccination. August 2012 - HPV Vaccine Now Recommended for Boys and Young Men
Help parents understand why boys should start the HPV vaccine series at age 11-12 years. CDC Expert Commentary, March 2012 - Clarifying Meningococcal Booster Dose Recommendations
In this video commentary from the CDC, Dr. Amanda Cohn clarifies the meningococcal booster dose recommendations for adolescents. CDC Expert Commentary, January 2012 - Increasing HPV Vaccine Coverage
In this video commentary from the CDC, Dr. Anne Schuchat outlines the steps that providers can take to ensure that cervical cancer does not develop in this generation of girls at the rates of their mothers and grandmothers. CDC Expert Commentary, December 2011 - Syncope After Vaccination
- Make Every Injection Safe!
In this video commentary from the CDC, Dr. Joseph Perz clears up myths and misperceptions that could be putting your patients at risk. CDC Expert Commentary, February 2011 - Vaccine and Disease Specific
Images and logos on this website which are trademarked/copyrighted or used with permission of the trademark/copyright or logo holder are not in the public domain. These images and logos have been licensed for or used with permission in the materials provided on this website. The materials in the form presented on this website may be used without seeking further permission. Any other use of trademarked/copyrighted images or logos requires permission from the trademark/copyright holder...more
![]() This graphic notice means that you are leaving an HHS Web site. For more information, please see the Exit Notification and Disclaimer policy.
This graphic notice means that you are leaving an HHS Web site. For more information, please see the Exit Notification and Disclaimer policy.
Contact Us:
- Centers for Disease Control and Prevention
1600 Clifton Rd
Atlanta, GA 30333 - 800-CDC-INFO
(800-232-4636)
TTY: (888) 232-6348 - New Hours of Operation
8am-8pm ET/Monday-Friday
Closed Holidays - cdcinfo@cdc.gov