Structure of Key Enzyme in Plague Bacterium FoundFrom NIST Tech Beat: August 17, 2006
Contact: Michael Baum (301) 975-2763
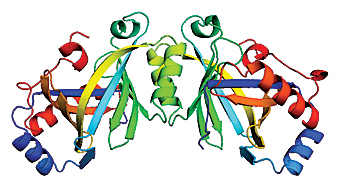
Researchers at the National Institute of Standards and Technology (NIST) have solved the structure of a key enzyme from the bacterium responsible for plague, finding that it has a highly unusual configuration. The results may shed light both on how the bacterium kills and on fundamental cell signaling processes. The NIST team determined the three-dimensional shape of class IV adenylyl cyclase (AC), an enzyme found in plague bacteria—Yersinia pestis—by purifying and crystallizing the protein and using X-ray crystallography at the Center for Advanced Research in Biotechnology to resolve its configuration. Adenylyl cyclase is a fundamental enzyme found in one form or another in organisms ranging from bacteria to mammals. It synthesizes cyclic AMP (cAMP*), an important signaling molecule that in turn triggers a variety of cellular processes. Six distinct classes of AC are known, playing a wide variety of roles. AC-II is part of the anthrax bacterium’s killing mechanism, for example, while AC-III triggers adrenaline release in humans. Shape plays an essential role in determining the biological function of a protein, but it’s very difficult to determine for such large molecules. Three-dimensional structures are known for only two other forms of AC. The NIST experiments revealed that AC-IV has a shape completely different from the other two known shapes. AC-IV folds into a rare form of a barrel-like shape previously seen in only three other unrelated proteins. The purpose of AC-IV in plague is not well understood, but it may play a role in disrupting cell processes in the infected host. Plague is not as common as it was in the Middle Ages, when it killed millions, but the World Health Organization still logs about 1,000 to 3,000 cases a year, an average of 10 to 15 in the United States. It is rated as a highest category biothreat agent by the Centers for Disease Control and Prevention and the National Institute of Allergy and Infectious Diseases. Fundamental molecular data on this enzyme and its various forms may be critical to the development of defenses against plague and other pathogens, including Bacillus anthracis (Anthrax) and Bordetella pertussis (Whooping cough). Beyond that, structural and functional studies of AC-IV, with its unusual shape, may lead to deeper understanding of the cAMP signaling mechanism and other fundamental cellular processes. Details of the structure of AC-IV are published in: D.T. Gallagher, N. Smith, S-K Kim, A. Heroux, H. Robinson and P. Reddy. Structure of the class IV adenylyl cyclase reveals a novel fold. J. Mol. Biol., In Press, Corrected Proof, Available online 14 Aug. 2006. *cyclic adenosine 3’, 5’- monophosphate |