For Consumers
FDA Advises Women With Breast Implants
| Please share copies of this printer-friendly PDF (963 KB) |
| Get the following and other FDA images on Flickr. |
 Binita Ashar, M.D., F.A.C.S., is one of about two dozen FDA scientists who are gathering information about a rare form of lymphoma in women with breast implants. They want to know why some of the women develop anaplastic large cell lymphoma (ALCL) and others do not. |
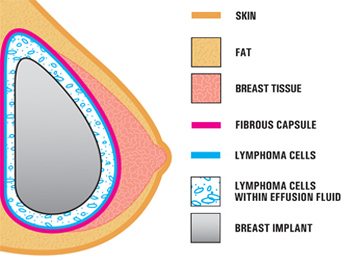 This illustration shows the typical location of the ALCL that was diagnosed in patients with breast implants. ALCL is lymphoma, a type of cancer involving cells of the immune system. It is not cancer of the breast tissue. |
 Get Consumer Updates by E-mail
Get Consumer Updates by E-mail
After an intensive review of known cases of a rare form of cancer in breast implant recipients, the Food and Drug Administration (FDA) says women with implants may have a very small, but increased risk of developing anaplastic large cell lymphoma, or ALCL.
FDA scientists reached that conclusion after examining scientific literature that focused on cases of ALCL in 34 women with breast implants, as well as information from agency reports, international regulatory agencies, scientific experts, and breast implant manufacturers.
But with an estimated five to 10 million breast implant recipients worldwide, agency experts say the known ALCL cases are too few to say conclusively that breast implants cause the disease. FDA believes there are about 60 of these ALCL cases worldwide, though that number is difficult to verify because not all of them were chronicled in scientific publications and some reports may have been duplicated.
In an effort to gather more information, FDA and the American Society of Plastic Surgeons are establishing a registry of ALCL patients who have breast implants. FDA scientists hope the registry yields enough information to better understand what the risks for developing ALCL are for women with breast implants.
In the meantime, Binita Ashar, a physician and FDA scientist evaluating ALCL cases, wants women with implants to have the latest information available about the risks. Ashar hopes her answers to three key questions will reassure women:
Q: What does FDA want women to know about breast implants and ALCL?
A: Although the risk is quite small, we want women to be aware that there have been reports of ALCL occurring around saline and silicone gel-filled breast implants. In the cases reported, ALCL was typically diagnosed years after the implant surgery. In most of these cases, the women were diagnosed after they observed changes in the look or feel of the area surrounding the implant.
Q: What advice is FDA giving to women?
A: If a woman with breast implants has no symptoms, FDA does not recommend doing anything additional. Women should continue monitoring their implants and obtaining regular breast screening evaluations. FDA does not recommend removing the implants.
Women who see changes in the way the area around the implant looks or feels—including swelling or pain around the implant—should see a physician for evaluation.
Women considering breast implants should be aware of the very small, but increased risk of developing ALCL and discuss it with a physician.
Q: How long will it be until FDA has more information to share?
A: Depending upon the number of reports the registry receives, we may have information that we can share soon. If we receive only a few reports, it may take some time to get the information we’re looking for—such as whether this is a new type of ALCL and if the type of implant has an impact on ALCL development.
Because the risk of developing ALCL is very small, the existing data support the continued marketing and use of breast implants. FDA will provide updates as new information becomes available.
If you want to learn more about breast implants and ALCL, visit FDA’s Center for Devices and Radiological Health. If you don’t have Internet access, call 1-800-638-2041 or 301-796-7100, and FDA will send information to your home.
If you have breast implants and have been diagnosed with ALCL, submit an online report through the agency’s MedWatch program online or call 1-800-332-1088. All reports are confidential.
This article appears on FDA's Consumer Updates page, which features the latest on all FDA-regulated products.
January 26, 2011
For More Information
- Press Release: FDA review indicates possible association between breast implants and a rare cancer
Anaplastic Large Cell Lymphoma (ALCL) Breast Implants - Safety Communication: Reports of Anaplastic Large Cell Lymphoma (ALCL) in Women with Breast Implants
- White Paper - Anaplastic Large Cell Lymphoma (ALCL) In Women with Breast Implants: Preliminary FDA Findings and Analyses
Questions and Answers about Anaplastic Large Cell Lymphoma (ALCL) Surveillance, Epidemiology, and End Results (SEER) Cancer.gov - Breast Cancer - PubMed.gov - Anaplastic Large-Cell Lymphoma in Women with Breast Implants
- PubMed.gov - Silicone Implant and Primary Breast ALK1-Negative Anaplastic Large Cell Lymphoma