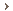Genomics in Action: Leslie G. Biesecker, M.D.
 It was a case that defied medical researchers for more than 40 years. An African-American family was being haunted from generation to generation by a mysterious, devastating disorder. It first appeared in 1937, when a baby boy was born to them with no eyes. As the baby grew into an infant, he was severely mentally and developmentally delayed, and had other physical abnormalities, including an unusually small head, and abnormalities of the teeth, ears, fingers, toes and heart. The mysterious disease struck again in this same extended family in the early 1950s and again in the 1960s, when three male cousins were born with a similar disorder. By now, some family members were terrified to have any more children.
It was a case that defied medical researchers for more than 40 years. An African-American family was being haunted from generation to generation by a mysterious, devastating disorder. It first appeared in 1937, when a baby boy was born to them with no eyes. As the baby grew into an infant, he was severely mentally and developmentally delayed, and had other physical abnormalities, including an unusually small head, and abnormalities of the teeth, ears, fingers, toes and heart. The mysterious disease struck again in this same extended family in the early 1950s and again in the 1960s, when three male cousins were born with a similar disorder. By now, some family members were terrified to have any more children.
The disorder was so devastating and its cause so mysterious that three separate teams of medical researchers made detailed studies of the family beginning in the mid-1960s. The first team of investigators found that the disorder was inherited and X-linked - which means only males had the full-blown condition because they have only one X chromosome - but they could get no further. Two more teams of investigators - one in the 1970s and another in the 1980s - attempted to identify the underlying biochemical or molecular cause of this disorder in the same family, but, again, were forced to give up when they ran out of leads.
It would take almost 20 more years to finally solve the mystery. That was when Leslie G. Biesecker, M.D., senior investigator and head of the Human Development Section of the National Human Genome Research Institute?s (NHGRI) Genetic Disease Research Branch, and his colleagues, joined the hunt.
"Genetic markers and maps just weren't good enough back then," explained Dr. Biesecker, a pediatrician and medical geneticist, on why earlier investigations had failed. "But, with data from the Human Genome Project (HGP), it's amazing how simple it was for us to find the causative genetic mutation. In fact, we were able to accomplish this task in mere months."
 Dr. Biesecker's laboratory conducts clinical and basic science research on a range of human developmental syndromes that exhibit various combinations of central nervous system, skeletal and abdominal organ "malformations." Another common characteristic of these malformation disorders is the presence of extra fingers or toes (polydactyly). He and his coworkers also study segmental overgrowth disorders, such as Proteus syndrome, a condition characterized by a variety of skin lesions and severe, uneven overgrowth of various parts of the body, particularly the limbs, hands and feet.
Dr. Biesecker's laboratory conducts clinical and basic science research on a range of human developmental syndromes that exhibit various combinations of central nervous system, skeletal and abdominal organ "malformations." Another common characteristic of these malformation disorders is the presence of extra fingers or toes (polydactyly). He and his coworkers also study segmental overgrowth disorders, such as Proteus syndrome, a condition characterized by a variety of skin lesions and severe, uneven overgrowth of various parts of the body, particularly the limbs, hands and feet.
In fact, Dr. Biesecker is one of the world's leading experts on such conditions, and patients are referred to his laboratory from all over the globe. Thus, in 2001, when a clinical genetics fellow, training in the NHGRI Medical Genetics program at Children's National Medical Center (CNMC) in Washington, D.C., approached Dr. Biesecker for help with a young infant born without eyes and with severe developmental delays, he and his colleagues were ready to spring into action.
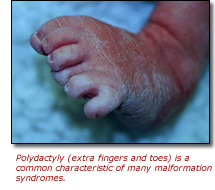
 Working with CNMC's Dr. Cynthia Tifft, Dr. Biesecker's group quickly rediscovered the family's medical history and interviewed the child's extended family members. They found that there had been six affected males over five generations in this family with varying degrees of abnormally small eyes (microphthalmia), small heads (microcephaly), mental retardation and heart and kidney problems. Armed with a wealth of data on the sequence of tens of thousands of human genes from the nearly completed HGP, Dr. Biesecker's laboratory then conducted a linkage analysis and a scan of the X-chromosome to narrow down the region where the culprit gene or genes for this condition, known as Lenz microphthalmia, might reside.
Working with CNMC's Dr. Cynthia Tifft, Dr. Biesecker's group quickly rediscovered the family's medical history and interviewed the child's extended family members. They found that there had been six affected males over five generations in this family with varying degrees of abnormally small eyes (microphthalmia), small heads (microcephaly), mental retardation and heart and kidney problems. Armed with a wealth of data on the sequence of tens of thousands of human genes from the nearly completed HGP, Dr. Biesecker's laboratory then conducted a linkage analysis and a scan of the X-chromosome to narrow down the region where the culprit gene or genes for this condition, known as Lenz microphthalmia, might reside.
After isolating and sequencing the suspect region from affected individuals, they identified a single DNA base substitution in the gene encoding BCL-6-interacting corepressor (BCOR) on the X chromosome. Subsequently, they found the same gene was implicated in a similar, but more severe developmental disorder known as oculofaciocardiodental syndrome (OFCD). Like Lenz microphthalmia, OFCD is an X-linked condition characterized by microphthalmia, congenital cataracts, and abnormalities affecting the heart, teeth, fingers and toes. However, unlike Lenz microphthalmia, which causes severe symptoms in males and no signs of the disorder in females who carry the altered gene, OFCD causes prenatal death in males and severe physical and mental disorders in females who carry one copy of the mutated gene.
Due to OFCD's striking clinical resemblance to Lenz microphthalmia, Dr. Biesecker and his colleagues investigated whether the mutations in the same gene on the X chromosome might be responsible for both of these conditions. What they found was that instead of single base mutations, individuals from seven OFCD-affected families had a variety of more severe mutations in the BCOR gene. Furthermore, they found that the more severe the mutations in this gene, the more severe were the clinical manifestations.
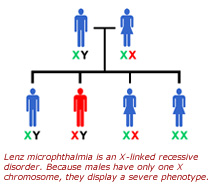
 With this evidence in hand, Dr. Biesecker's group identified and cloned a zebrafish version of the BCOR gene. In subsequent experiments, in which they suppressed the expression of, or "knocked-down," BCOR gene in zebrafish using morpholino technology (see sidebar), they were able to replicate severe developmental disruptions of the eye, skeleton and central nervous system of zebrafish consistent with Lenz microphthalmia and OFCD. According to Dr. Biesecker, these findings suggest that BCOR is a key transcriptional regulator during early embryogenesis.
With this evidence in hand, Dr. Biesecker's group identified and cloned a zebrafish version of the BCOR gene. In subsequent experiments, in which they suppressed the expression of, or "knocked-down," BCOR gene in zebrafish using morpholino technology (see sidebar), they were able to replicate severe developmental disruptions of the eye, skeleton and central nervous system of zebrafish consistent with Lenz microphthalmia and OFCD. According to Dr. Biesecker, these findings suggest that BCOR is a key transcriptional regulator during early embryogenesis.
"We believe it is the job of the normal product of this gene to repress the transcription of other genes involved in normal embryo development," explained Dr. Biesecker. "Although BCOR also is known to function with the white blood cell gene BCL-6 - a gene that frequently is misregulated in B-cell lymphomas - that function has nothing to do with these two diseases. This means that the BCOR gene has a critical role in the development of many parts of the body and not just white blood cells. This wide ranging importance was completely unknown prior to these findings."
Dr. Biesecker and his group found that mutations in BCOR cause something to be overexpressed, or "upregulated." What that "something" is remains to be determined. 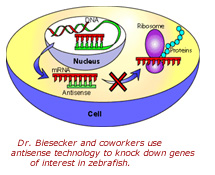 To identify which gene or genes are being affected by the loss of BCOR, Dr. Biesecker's group is using microarray technology to screen for misregulated genes in BCOR-depleted zebrafish. Using this approach, they recently identified a candidate gene for the heart defects that are common in Lenz microphthalmia patients. Dr. Biesecker believes that at least several more genes are involved in the clinical symptoms of these disorders. Experiments currently are underway with NHGRI investigators Dr. Shawn Burgess and Dr. Benjamin Feldman to find those additional genes.
To identify which gene or genes are being affected by the loss of BCOR, Dr. Biesecker's group is using microarray technology to screen for misregulated genes in BCOR-depleted zebrafish. Using this approach, they recently identified a candidate gene for the heart defects that are common in Lenz microphthalmia patients. Dr. Biesecker believes that at least several more genes are involved in the clinical symptoms of these disorders. Experiments currently are underway with NHGRI investigators Dr. Shawn Burgess and Dr. Benjamin Feldman to find those additional genes.
In the meantime, Dr. Biesecker's findings are already making a difference in the lives of the family whose members are affected by Lenz microphthalmia. Several family members previously made the decision to forgo having children based on the potential risk of their passing on the altered gene to their offspring. However, that is not an optimal outcome, said Dr. Biesecker, because these individuals may not, in fact, carry the altered gene. Recently, his laboratory developed a genetic test to identify BCOR mutations and has made it available to family members, several of whom are currently considering using the test. Thus, although the family must still carry the burden of this genetic illness, it is a mystery no longer.
Last Reviewed: March 13, 2012