Genomics in Action: Elizabeth G. Nabel, M.D.
Seeking To Improve Coronary Stent Therapy Through Genetic Profiling
 Coronary heart disease is the leading cause of death in the United States and
other industrialized countries. To improve the outlook for the 13 million Americans suffering from this common disorder, a laboratory that recently joined the National
Human Genome Research Institute (NHGRI) is tapping into the power of genomics
to develop better therapies.
Coronary heart disease is the leading cause of death in the United States and
other industrialized countries. To improve the outlook for the 13 million Americans suffering from this common disorder, a laboratory that recently joined the National
Human Genome Research Institute (NHGRI) is tapping into the power of genomics
to develop better therapies.
A leader in the effort to apply genetic and genomic tools to the treatment of cardiovascular disease, Elizabeth G. Nabel, M.D., was named director of the National Heart, Lung, and Blood Institute (NHLBI) in January 2005. In conjunction with this appointment, her own laboratory was relocated to NHGRI's Division of Intramural Research, with its focus on using genomics to advance human health.
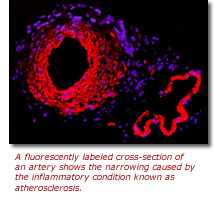 Coronary heart disease is primarily the result of a chronic inflammatory condition,
called atherosclerosis, which causes a progressive build up of fat, cholesterol
and other substances that narrow the heart's blood vessels, in particular the
coronary arteries. If the arteries narrow to the point that blood cannot flow
freely, a heart attack may occur.
Coronary heart disease is primarily the result of a chronic inflammatory condition,
called atherosclerosis, which causes a progressive build up of fat, cholesterol
and other substances that narrow the heart's blood vessels, in particular the
coronary arteries. If the arteries narrow to the point that blood cannot flow
freely, a heart attack may occur.
Over the past several decades, many patients with badly narrowed, or stenosed, coronary arteries have been treated by balloon angioplasty followed by stenting. In this two-step procedure, doctors make a small incision and thread a deflated balloon into a patient's narrowed arteries. When the balloon is inflated, the arteries are widened and a stent, which is a wire-mesh tube that looks like a small spring, is inserted to keep the arteries propped open on a long-term basis.
Angioplasty and stenting are significantly less invasive and risky than open-heart surgery, and have proven an effective alternative for reducing and preventing deaths from heart attacks. However, a sizable fraction of the more than 1 million patients who undergo coronary angioplasty and stenting each year in the United States develop restenosis - a re-narrowing of the widened segment of the artery. Doctors often have to perform another procedure on patients to re-open the arteries. As a result, these individuals can experience additional complications and are at a significantly higher risk of a repeat heart attack and death.
The high rate of restenosis associated with stents has led cardiovascular disease researchers like Dr. Nabel, who is now a senior investigator in NHGRI's Genome Technology Branch, to investigate the genetic determinants of this phenomenon. In particular, she and her coworkers are interested in the role that variants in genes controlling inflammation and cell proliferation play in restenosis. They also are investigating therapeutic options for bringing this process back into balance.
"About 25 percent of stented patients develop restenosis. No one knows exactly why this happens," said Dr. Nabel. "However, we believe, based on our research, that genetic susceptibility to inflammation and excessive cell proliferation is a significant risk factor for developing restenosis. If we can intervene in that process, we may be able to significantly improve the effectiveness of coronary stents."
 To test their hypothesis, Dr. Nabel's laboratory - in conjunction with researchers
at NHLBI - is following a group of more than 450 patients who received "bare
metal" coronary artery stents to better understand the molecular mechanisms
of restenosis and the genetic markers that may help to predict it. Specifically,
they have collected blood samples from all of the study participants and are
performing a variety of genetic analyses. Ultimately, they hope to identify
the genetic differences between those who develop restenoisis versus those who
do not develop.
To test their hypothesis, Dr. Nabel's laboratory - in conjunction with researchers
at NHLBI - is following a group of more than 450 patients who received "bare
metal" coronary artery stents to better understand the molecular mechanisms
of restenosis and the genetic markers that may help to predict it. Specifically,
they have collected blood samples from all of the study participants and are
performing a variety of genetic analyses. Ultimately, they hope to identify
the genetic differences between those who develop restenoisis versus those who
do not develop.
"We are developing gene, RNA and protein profiles of these individuals to try to establish who is most likely to develop in-stent restenosis. If we are successful, we can begin to stratify patients based upon genetic risk, and tailor therapies specifically for each group," explained Dr. Nabel.
In-stent restenosis usually occurs within six to nine months after stenting. However, there is typically little progression after that. Because of this, Dr. Nabel and colleagues believe they can permanently eliminate in-stent restenosis by developing drug-eluting stents that release locally inhibitors of inflammation and cell proliferation for the first few months after the procedure.
Although several drug-eluting stents are currently approved by the Food and Drug Administration for preventing restenosis, the current ones emit drugs that tend to have non-specific action and, thus, potentially serious side effects. Dr. Nabel hopes to find ways to further reduce the side effects of restenosis drugs by better understanding the underlying inflammatory and cell-proliferation process and by developing more targeted therapeutic agents. In particular, her group is looking for substances that may be able to switch off the inflammatory process until the stent has fully settled in.
Recent studies have shown that substances called cyclins and cyclin-dependent kinases play important roles in the normal repair of vascular and cardiac tissue following injury. Furthermore, in a paper published in the Journal of Clinical Investigation in August 2004 (Bone marrow¿derived immune cells regulate vascular disease through a p27Kip1-dependent mechanism), Dr. Nabel's group reported the discovery a unique cyclin-dependent kinase inhibitor, called p27Kip1, which appears to be a major regulator of vascular smooth muscle cell proliferation during normal arterial injury repair. p27Kip1 affects the proliferation and migration of immune cells from the bone marrow to the sites of arterial injuries.
"In patients who experience restenosis, it is possible that regulation of the repair process by p27Kip1 is somehow defective, allowing unchecked cell proliferation," said Dr. Nabel.
Recently, her laboratory identified a nuclear protein that binds to p27Kip1, which may be a good target for therapy. In fact, by using a small interfering RNA molecule to suppress gene expression and thereby deplete cells of this nuclear protein, the researchers were able to inhibit the uncontrolled cell proliferation seen with restenosis. The findings suggest the possibility of coating a stent with a polymer that would release a drug capable of altering p27Kip1 expression, thereby influencing the restenosis process.
Before any of this can occur, however, Dr. Nabel said the results of her group's ongoing in-stent restenosis study need to be validated in another group of patients. "We believe the data we are generating, if confirmed in a larger trial, can be used in several ways: first, to discover new genes and pathways integral to the pathophysiology of in-stent restenosis; second, to use these discoveries to develop new targets for drug-eluting stents; third, to predict who will develop in-stent restenosis; and fourth, to determine which patients are the best candidates for drug-eluting stents.
"We are also very keen to use these discovery approaches to identify disease-causing genes and to develop new therapeutic targets for helping those individuals who adversely respond to coronary stenting with an intense inflammatory and proliferative reaction."
Last Reviewed: March 13, 2012