Genomics in Action: Pu Paul Liu, M.D., Ph.D.
Getting Small to Cure Leukemia
By Jim Swyers, NHGRI Staff Writer
 It is an exquisitely orchestrated and complicated system that, when functioning normally, leads to the formation and development of all the blood cells that keep us healthy. But as the National Human Genome Research Institute's (NHGRI) Pu Paul Liu, M.D., Ph.D. and other researchers pursuing the genetic roots of leukemia know all too well, if even a single part of this system goes haywire, the results can be devastating.
It is an exquisitely orchestrated and complicated system that, when functioning normally, leads to the formation and development of all the blood cells that keep us healthy. But as the National Human Genome Research Institute's (NHGRI) Pu Paul Liu, M.D., Ph.D. and other researchers pursuing the genetic roots of leukemia know all too well, if even a single part of this system goes haywire, the results can be devastating.
Dr. Liu's laboratory studies the genetic control of hematopoiesis (pronounced
HE-mehtopo-E-sis), which is the process through which special cells in the bone
marrow, called hematopoietic stem cells, differentiate into all of the types
of mature cells that circulate in the bloodstream. These include the red blood
cells that carry oxygen and other materials to tissues of the body, the white
blood cells that fight infection and disease, and the platelets that help prevent
bleeding by causing blood clots to form.
"Leukemia is an example of hematopoiesis gone awry," explained Dr.
Liu, a senior investigator in the Genetics and Molecular Biology Branch of NHGRI's
Division of Intramural Research. "When this occurs, the body produces large
numbers of abnormal blood cells that cause a chain reaction of events, which
are often catastrophic to the blood, immune and other systems."
 His group is especially interested in understanding how disturbances in hematopoiesis
lead to acute myeloid leukemia (AML), one of the most common types of leukemia
in humans. In fact, he directs one of the leading laboratories in the world
investigating the underlying genetic causes of and potential therapies for AML.
As a result of recent advances in understanding the genetics of AML, his group
is now using a "rational design" approach to develop potential new
therapies that might prove more specific and less likely to cause complications
compared to current therapies.
His group is especially interested in understanding how disturbances in hematopoiesis
lead to acute myeloid leukemia (AML), one of the most common types of leukemia
in humans. In fact, he directs one of the leading laboratories in the world
investigating the underlying genetic causes of and potential therapies for AML.
As a result of recent advances in understanding the genetics of AML, his group
is now using a "rational design" approach to develop potential new
therapies that might prove more specific and less likely to cause complications
compared to current therapies.
AML, which strikes about 12,000 Americans each year, usually occurs in people
older than 25. It is considered a cancer of the bone marrow - the spongy tissue
inside the large bones of the body - and usually has a very rapid progression
if not treated.
Although 60 to 70 percent of patients with AML experience complete remission
after intensive chemotherapy treatment, more than 50 percent of those individuals
eventually develop AML again, or relapse. In relapsed cases, hematopoietic stem
cell transplantation (HSCT) may be the next course of treatment. However, HSCT
often fails because of either relapses or the rejection of the transplanted
cells by the patient's own immune system.
Radiation therapy, which employs x-rays or other high-energy rays, also is
used for treating AML, particularly if the leukemic cells have spread to other
organs and tissues, such as the brain. Unfortunately, radiation often damages
healthy tissue in addition to the cancerous cells.
Thus, despite important advances in the therapy of AML, the majority of patients (approximately 80 percent) will eventually die from the disease within five years of diagnosis. The shortcomings of current approaches are why Dr. Liu's laboratory, along with many other laboratories around the world, are seeking a better understanding of the molecular pathogenesis of AML and other leukemias.
"None of the current therapies for AML are optimal," said Dr. Liu. "We desperately need more targeted approaches that treat the underlying causes of this devastating disease without harming the patient."
Many leukemias begin when a piece of one chromosome becomes detached and reattaches to the same chromosome or another chromosome. This process, which is called inversion if it involves the same chromosome or translocation if it involves a different chromosome, can bring together two unrelated genes. The result, referred to as gene fusion, can wreak havoc on the cell.
Various studies have shown that some of these fused genes produce proteins that deliver a double whammy: First, they turn off genes that normally direct hematopoietic differentiation, and then, they activate genes that help the cells proliferate. The result is uncontrolled growth of immature cells.
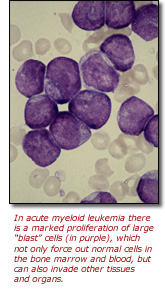
In the case of AML, an inversion on chromosome 16 causes accumulation of immature myeloid progenitors, or the precursors of white blood cells. These abnormal blood cells (also called leukemia cells or blasts) not only are unable to do their usual job, but they also multiply abnormally and build up in the bone marrow and blood. This leaves less room for healthy white blood cells, red blood cells and platelets to flourish, leading to infection, anemia and uncontrolled bleeding. In addition, the blasts often spread to other parts of the body, including the brain, spinal cord, skin and gums, doing extensive damage in those organs and tissues.
Dr. Liu was the first to demonstrate that the AML-associated genetic inversion on chromosome 16 generates a fusion gene, called CBFB-MYH11. Furthermore, when inserted into mice, the CBFB-MYH11 fusion gene blocks normal hematopoiesis and makes the animals susceptible to leukemia.
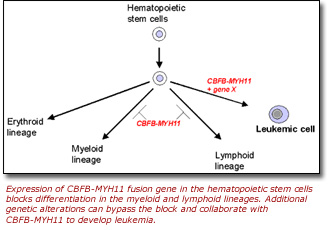 Dr. Liu's laboratory has gone on to find that although the expression of the
CBFB-MYH11 gene is the key initiator of AML, other genes also play a
role in the disease. In a recent study, Dr. Liu and collaborators from the National
Cancer Institute and the University of Massachusetts Medical School reported
the identification of dozens of additional genes that specifically cooperate
with the CBFB-MYH11 gene in the development of AML.
Dr. Liu's laboratory has gone on to find that although the expression of the
CBFB-MYH11 gene is the key initiator of AML, other genes also play a
role in the disease. In a recent study, Dr. Liu and collaborators from the National
Cancer Institute and the University of Massachusetts Medical School reported
the identification of dozens of additional genes that specifically cooperate
with the CBFB-MYH11 gene in the development of AML.
More importantly, however, they showed that as few as one additional alteration
(a second genetic "hit") accelerated the development of leukemia in
their mouse model containing the fusion gene. Of particular note was the identification
of two genes encoding "zinc finger transcription factor" proteins,
Plag1 and Plagl2, which also appear to be over-expressed in AML.
"We now know that other genes are involved in the development and progression
of AML," said Dr. Liu. "This allows us to search for all of the downstream
target genes of the fusion protein."
While additional genes that contribute to AML continue to be identified on
a regular basis, Dr. Liu's laboratory is beginning to develop and test new approaches
for treating AML. Based on early but promising results, his laboratory recently
received a five-year grant from The Leukemia and Lymphoma Society to develop
approaches to treating two subtypes of AML, as well as chronic myeloid leukemia.
For this effort, his group is collaborating with a structural biologist and a molecular chemist to design "small molecules" that will specifically interfere with the activity of the AML fusion protein. These small molecules should be easier to deliver, cheaper to synthesize, and less likely to trigger an immune response compared to current AML therapies, such as those using monoclonal antibodies.
"Based on our studies, we understand that certain protein-protein interactions are key to the development of AML and other leukemias. Small molecules that specifically interrupt these protein-protein interactions might restore normal hematopoietic function without the toxic or immunogenic side effects encountered with other therapeutic approaches. Ultimately, we believe that such small molecule therapies for AML will be most effective when combined with other treatments," said Dr. Liu.
Last Reviewed: August 2006