Reduce
new cases of chronic kidney disease and its complications, disability, death, and economic costs.
Chronic kidney failure is the most significant result of
chronic kidney disease. When kidney function has deteriorated and is no longer
adequate to sustain life and the process is considered irreversible, renal
replacement therapy (RRT)—dialysis or transplantation—becomes necessary to
maintain life. Treated chronic kidney failure, also called end-stage renal
disease (ESRD), is the most feared consequence of kidney disease. Chronic renal
insufficiency, however, is more common than treated chronic kidney failure and
can also severely affect health and well-being. Therefore, ideally, programs
should be directed at preventing the development of chronic renal insufficiency
and its subsequent progression to ESRD.
Unfortunately, chronic renal insufficiency is usually asymptomatic, and the
exact number of people affected is unknown. The best available estimates are
based on national surveys. Current estimates indicate approximately 10 million
persons aged 12 years and older have some form of chronic kidney disease.[1]
People with end-stage kidney failure represent a small fraction of all
individuals with chronic kidney disease. A significant proportion of people
with chronic kidney failure progress to end stage. The challenge is to initiate
effective programs to prevent progression of established kidney disease and to
institute methods to assess the progress of such initiatives.
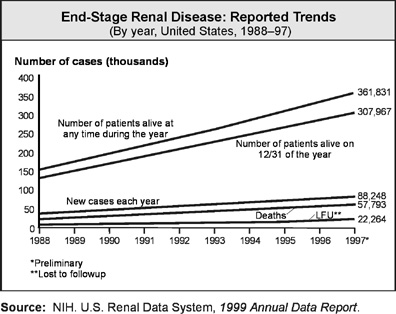
In 1997, 80,248 new cases of end-stage kidney failure were
reported.[2]
Virtually all of these patients became permanently dependent on renal
replacement therapy to stay alive.
Dialysis and kidney transplantation are the two methods of
treatment available to people with kidney disease when they reach end stage. In
1997, 361,031 people in the United States depended on either dialysis or
transplants to replace the function of their own failed kidneys.[3]
Although these treatments are lifesaving, dialysis and transplants have
substantial limitations. Neither treatment restores normal health, and both are
expensive.[4]
The rates of illness, disability, and death experienced by individuals with
treated chronic kidney failure are substantially higher than those of the
general population.[5]
In most instances, terminal kidney failure develops as the
result of progressive damage to the kidneys over a decade or more. A number of
underlying diseases can cause progressive kidney failure. The two most
important of these are diabetes, which in 1997 accounted for 42 percent of the
new cases of chronic kidney failure, and high blood pressure, which was responsible
for 26 percent of the new cases.[6] Other conditions that contribute
significantly include glomerulonephritis, vasculitis, interstitial nephritis,
and genetic and congenital disorders, particularly polycystic kidney disease.[7]
Chronic kidney failure affects people of all ages. The
number of new cases peaks in the sixth decade of life, but 25 percent of
persons arriving at end stage in 1997 were under age 45 years, and 1.5
percent—nearly 1,100—were under age 20 years.6 Kidney failure is particularly devastating in
childhood, often resulting in impaired growth and development.
A worrisome increase in the number of new cases of kidney
failure occurred between 1987 and 1997. The rate increased from 142 per million
population in 1987 to 296 per million population in 1997,[8] representing an increase in the
annual number of new cases from 34,797 to 80,248,[9]
respectively.
This relentless growth in new cases of kidney failure has
occurred in spite of the fact that death rates from other diseases, especially
cardiovascular diseases, have declined.[10]
The increase has not been confined to a single age group. Although the rates of
new cases have grown slightly more rapidly for individuals aged 75 years and
older, sizable increases have been noted in every age group.[11]
The causes of these increases are not completely understood,
but one major factor appears to be an increase in the number of new cases of
diabetes, particularly type 2 diabetes.[12], [13]
In 1987, the rate of new cases of treated chronic kidney failure due to
diabetes was 45 per million population. By 1997, the rate had increased to 124
per million population.[14]
Treatment for end-stage kidney failure has a substantial
impact on Federal resources for health care. The 1972 Social Security Amendment
(Public Law 92-603) instituted federally financed health care coverage for
dialysis and renal transplantation, effective July 1, 1973. The cost of this
program has far exceeded original expectations. Medicare spending in 1996 was estimated
to be $10.96 billion, a 12.5 percent increase from the $9.74 billion spent in
1995. The total expenditure by all payers for treating these patients in 1996
was estimated at $14.55 billion, up from $13.05 billion in 1995.[15]
Although this patient population made up only 0.6 percent of the total Medicare
population in 1994, it consumed 5.1 percent of Medicare expenditures.[16]
The increases in the cost per patient have been modest, but the driving force
behind the growth in these expenditures has been the growing number of
patients.
Kidney disease develops and progresses more rapidly to end
stage in people with chronic health problems (such as type 1 or type 2 diabetes
or high blood pressure) or with a family history of genetic kidney diseases.
Therefore, people with these chronic health problems require counseling about
the possibility of kidney disease and the steps they must take to avoid serious
kidney complications. Also, people who have proteinuria and/or elevated serum
creatinine have a greater likelihood of developing serious cardiovascular
disease (CVD) complications. Therefore, cardiovascular risk assessment and
management should include kidney function to prevent the consequences of kidney
failure. Because national data systems will not be available in the first half
of the decade to track progress, these issues are not addressed in the chapter.
Kidney disease has a disproportionate impact on certain
racial and ethnic groups, especially African Americans and American Indians or
Alaska Natives. African Americans have the highest overall risk of chronic
kidney disease. The reasons are not entirely explained by the higher number of
persons in this population who have diabetes and high blood pressure.[17], [18]
On average, African Americans develop end-stage kidney failure at an earlier
age than whites (55.8 years compared to 62.2 years).[19]
American Indians or Alaska Natives have a much higher risk of chronic kidney
disease due to diabetes than whites. Overall, the rates of new cases are 4
times higher in African Americans and American Indians or Alaska Natives and
1.5 times higher in Asians or Pacific Islanders than in whites.
Annual increases in ESRD rates are greater in certain racial
and ethnic populations than in white populations. Rates of new cases are
increasing by 7 percent per year for African Americans, 10 percent per year for
American Indians or Alaska Natives, and 11 percent for Asians or Pacific Islanders,
compared to 6 percent per year for whites. Two communities of an American
Indian Tribe, the Zuni Pueblo in New Mexico and in Sacaton, Arizona, may have
the highest rates of chronic kidney failure in the world, at 12.6 and 14.0
times the overall average U.S. rate, respectively. Projections indicate that
increases in the rates of new cases will continue in American Indians or Alaska
Natives.
Although complete data are not yet available, some evidence
indicates that persons of Mexican ancestry also may have a high risk of
developing chronic kidney failure, particularly due to diabetes.[20], [21]
In 1995, the Health Care Financing Administration changed the way in which data
on race and ethnicity are collected on the Medical Evidence Form used to enroll
patients into the Medicare End-Stage Renal Disease Program. Data from 1997
suggest that 7 percent of the ESRD patients are of Mexican ancestry and another
4 percent are of Hispanic ancestry from areas other than Mexico.[22]
The disproportionately high rates of chronic kidney failure
among certain racial and ethnic groups have resulted in a greater burden of
disease in these communities. In 1996, African Americans constituted 12.6
percent of the U.S. population but 29.8 percent of ESRD patients; American
Indians or Alaska Natives constituted 0.9 percent of the U.S. population but
1.7 percent of those receiving renal replacement therapy.[23]
On December 31, 1996, the point-prevalent rate per million population (adjusted
for age and gender) was 3,404 in African Americans and 2,761 in American
Indians or Alaska Natives, compared to 754 in whites, differences of 4.5- and
3.7-fold, respectively.[24]
Data on persons of Asian or Pacific Islander ancestry indicate slightly higher
incidence and prevalence rates than those for whites.[25]
There is a slight preponderance of kidney failure in men. In
1997, the incidence of treated chronic kidney failure was 322 per million
population in men, compared with 271 per million in women.8
Renal transplantation is an important lifesaving renal
replacement therapy and has been shown to offer many advantages when compared
with dialysis.[26], [27]
In 1997, 12,445 transplants were performed in the United States. There was
significant gender discrepancy, with 7,352 transplants for men, compared with
4,948 for women.[28] Racial and
ethnic disparities also exist. Between 1994 and 1997, the first cadaveric
transplantation rates (per 100 patient years) in the pediatric age group were
31 for black males, 28 for white males, 19 for black females, and 26 for white
females. For recipients between the ages of 20 and 44 years, the rates were 7
for black males, 17 for white males, 7 for black females, and 15 for white females.
In the 45- to 65-year age group, the rates were 4 for black males, 8 for white
males, 2 for black females, and 6 for white females.[29]
The data from the U.S. Renal Data System (USRDS) database also confirm that the
transplantation rate is lower for Native Americans. The transplantation rate in
Asians is equivalent to the rate in whites.[30]
Reasons for the racial and ethnic disparities in the rate of transplantation
are varied and include differences in finding human leukocyte antigen matches,
cultural attitudes and beliefs on the part of both patients and health care
providers, socioeconomic status, rates of organ donation, and geographic
location.
Major risk factors for the development and progression of
chronic kidney disease include diabetes, high blood pressure, environmental
exposures, proteinuria, family history of kidney disease, and increasing age.
African Americans and American Indians or Alaska Natives who have these risk
factors are especially susceptible to the development of chronic and
progressive kidney disease.[31], [32]
Strategies for preventing the development of chronic kidney disease, therefore,
should use appropriate methods to target these populations.
Under certain circumstances, the progression of kidney
disease to end stage can be slowed or halted. Three interventions are effective
in certain defined populations: glycemic control (for patients with diabetes),
blood pressure control (for patients with high blood pressure), and use of
angiotensin-converting enzyme (ACE) inhibitors. Interventions to slow the
progression of kidney disease and prevent chronic kidney failure are likely to
have the greatest impact if applied early in the course of the disease.
Unfortunately, because kidney disease in its early stages is generally
asymptomatic, many people who would benefit from these interventions are not
identified. Early identification of patients at risk for chronic kidney disease
is essential in reducing the growth in the number of new cases of treated
chronic kidney failure. For example, microalbuminuria screening and more
intensive treatment of patients with microalbuminuria are an important part of
a strategy to reduce nephropathy in persons with type 1 diabetes, both in terms
of economic indices and clinical outcomes.[33], [34],
[35]
This strategy also may be useful in type 2 diabetes.
Patient care must continue to emphasize interventions to
conserve residual renal function. At a certain stage, however, providing
appropriate preparation for renal replacement therapy becomes advisable.
Several studies show that many patients with chronic kidney failure do not
receive optimum preparation for treated chronic kidney failure in the year prior
to the commencement of RRT. This lack of optimal preparation has a substantial
effect on the cost of care and on illness and disability at the time of RRT.[36]
Kidney transplantation has emerged as the preferred therapy
for many patients with treated chronic kidney failure, particularly children.
Kidney transplantation confers a survival advantage over dialysis.[37]
Over the past decade, transplantation success rates, especially 1-year patient
and graft survival, have improved steadily. This improvement has been observed
in both cadaveric and living-related transplants.[38], [39]
For young children, kidney transplantation results in improved rates of growth.[40] Because of accumulating evidence on
the advantages of transplantation, equal access of all population groups to
transplantation is a substantial concern. Certain racial and ethnic groups and
women consistently have longer waiting times and lower rates of kidney
transplantation than white males.[41], [42], [43], [44],
[45],
[46]
Attention to risk factors for kidney disease and interventions
to slow its progression are urgently needed. This need is driven by the
increasing number of cases of treated chronic kidney failure, its
disproportionate effect on certain racial and ethnic groups, the high societal
cost of the disease, and the impact on Federal health care resources.
Healthy People 2000 did not include a chapter on chronic
kidney disease. However, objectives relating to chronic kidney disease were
included in several chapters. Since the mid-1980s, the number of new cases of
ESRD has grown steadily. One objective concerning diabetes addressed ESRD.
Results show that ESRD among people with diabetes has more than doubled since
1987 and is moving away from the target. Subobjectives tracking ESRD due to
diabetes among African Americans and American Indians or Alaska Natives also
are moving away from their targets.
Note: Unless
otherwise noted, data are from the Centers for Disease Control and Prevention, National Center for Health Statistics, Healthy People 2000 Review, 1998–99.
Chronic Kidney Disease
Goal: Reduce new cases of chronic kidney disease and its
complications, disability, death, and economic costs.
|
Number
|
Objective Short Title
|
|
4-1
|
End-stage renal disease
|
|
4-2
|
Cardiovascular disease deaths in
persons with chronic
kidney failure
|
|
4-3
|
Counseling for chronic kidney failure
care
|
|
4-4
|
Use of arteriovenous fistulas
|
|
4-5
|
Registration for kidney
transplantation
|
|
4-6
|
Waiting time for kidney transplantation
|
|
4-7
|
Kidney failure due to diabetes
|
|
4-8
|
Medical therapy for persons with
diabetes and proteinuria
|
Target: 217
new cases per million population.
Baseline: 289
new cases of end-stage renal disease per million population were reported in
1997.
Target setting
method: Better than the best.
Data source: U.S.
Renal Data System (USRDS), NIH, NIDDK.
|
Total Population, 1997
|
New Cases of End-Stage
Renal
Disease
|
|
Rate per Million
|
|
TOTAL
|
289
|
|
Race and ethnicity
|
|
American Indian or Alaska Native
|
586
|
|
Asian or Pacific Islander
|
344
|
|
Asian
|
DNC
|
|
Native Hawaiian and other
Pacific
Islander
|
DNC
|
|
Black or African American
|
873
|
|
White
|
218
|
|
|
|
Hispanic or Latino
|
DNA
|
|
Not Hispanic or Latino
|
DNA
|
|
Black or African American
|
DNA
|
|
White
|
DNA
|
|
Gender
|
|
Female
|
242
|
|
Male
|
348
|
|
Family income level
|
|
Poor
|
DNC
|
|
Near Poor
|
DNC
|
|
Middle/high income
|
DNC
|
|
Select populations
|
|
Age groups
|
|
Under 20 years
|
13
|
|
20 to 44 years
|
109
|
|
45
to 64 years
|
545
|
|
65
to 74 years
|
1,296
|
|
75
years and older
|
1,292
|
DNA = Data have not been analyzed. DNC = Data are not
collected. DSU = Data are statistically unreliable.
The current average annual
increase in new cases of treated chronic kidney failure rates is 6 percent.
Therefore, the expected rate in 2010 would be 612 new cases per million
population. Without improvements in prevention and because of changes in
demographics and increases in the number of cases of diabetes, rates of new
cases of treated chronic kidney failure are expected to continue to rise 5 to 8
percent per year.
Target: 52
deaths per 1,000 patient years at risk.
Baseline: 70
deaths from cardiovascular disease per 1,000 patient years at risk (in persons
with ESRD) occurred in 1997.
Target setting
method: Better than the best.
Data source: U.S.
Renal Data System (USRDS), NIH, NIDDK.
|
Persons With Treated
Chronic Kidney
Failure, 1997
|
Deaths
From
Cardiovascular Disease
|
|
Per 1,000 Patient Years at Risk
|
|
TOTAL
|
70
|
|
Race and ethnicity
|
|
American Indian or Alaska Native
|
63
|
|
Asian or Pacific Islander
|
60
|
|
Asian
|
DNC
|
|
Native Hawaiian and other
Pacific
Islander
|
DNC
|
|
Black or African American
|
62
|
|
White
|
75
|
|
|
|
Hispanic or Latino
|
DNA
|
|
Not Hispanic or Latino
|
DNA
|
|
Black or African American
|
DNA
|
|
White
|
DNA
|
|
Gender
|
|
Female
|
73
|
|
Male
|
67
|
|
Family income level
|
|
Poor
|
DNC
|
|
Near Poor
|
DNC
|
|
Middle/high income
|
DNC
|
DNA = Data have not been analyzed. DNC = Data are not
collected. DSU = Data are statistically unreliable.
Cardiovascular disease is
the major cause of death among patients with chronic renal failure and ESRD.
Therefore, targeting reduction in CVD deaths will lead to a significant
decrease in deaths for this population. The increased risk of CVD in kidney
disease patients is evident before the onset of terminal kidney failure. Increases
in the number of CVD deaths also are seen in individuals with proteinuria or
elevated creatinine (both are markers of declining kidney function). CVD death
rates in the treated chronic kidney failure population are estimated to be
30-fold higher than in the general population.[47]
The known risk factors for CVD in the general population include age, male
gender, diabetes, elevated cholesterol, high blood pressure, smoking, and
family history. Elevated homocysteine levels in the blood also may be an
important risk factor in treated chronic kidney failure patients and at earlier
stages in the progression of kidney disease.[48], [49],
[50]
Strategies to reduce CVD deaths should target risk reduction before terminal kidney
failure.[51] All responsible health care
providers can initiate the strategies to reduce CVD deaths as suggested in
published guidelines.51
Target: 60
percent.
Baseline: 45
percent of newly diagnosed patients with treated chronic kidney failure
received counseling on nutrition, treatment choices, and cardiovascular care in
1996.
Target setting
method: 33 percent improvement. (Better than the best will be used when
data are available.)
Data source:
U.S. Renal Data System (USRDS), NIH, NIDDK.
|
Newly Diagnosed Patients
With Treated Chronic Kidney Failure, 1996
|
Received
Counseling Prior to Renal
Replacement
Therapy
|
|
Percent
|
|
TOTAL
|
45
|
|
Race and ethnicity
|
|
American Indian or Alaska Native
|
DNA
|
|
Asian or Pacific Islander
|
DNA
|
|
Asian
|
DNC
|
|
Native Hawaiian and other
Pacific
Islander
|
DNC
|
|
Black or African American
|
DNA
|
|
White
|
DNA
|
|
|
|
Hispanic or Latino
|
DNA
|
|
Not Hispanic or Latino
|
DNA
|
|
Black or African American
|
DNA
|
|
White
|
DNA
|
|
Gender
|
|
Female
|
DNA
|
|
Male
|
DNA
|
|
Family income level
|
|
Poor
|
DNC
|
|
Near Poor
|
DNC
|
|
Middle/high income
|
DNC
|
DNA = Data have not been analyzed. DNC = Data are not
collected. DSU = Data are statistically unreliable.
Medically appropriate care
of kidney disease patients within 12 months before the start of renal
replacement therapy reduces the substantial illness, disability, and death
associated with treated chronic kidney failure.[52]
Appropriate preparation for RRT includes reduction in CVD risk factors,
treatment of anemia, optimum therapy to preserve residual renal function,
consultation about nutrition, and patient education about RRT methods. Patients
should be seen by a specialist in RRT at least 12 months prior to initiation of
RRT for general counseling. However, specific issues—such as vascular access and
estimation of residual renal function—need to be addressed at least 6 months
prior to RRT. Many patients with chronic renal failure are not seen by health
care professionals who have RRT expertise until very near the time that RRT
will be required. In a USRDS survey of 3,468 new dialysis patients, 55 percent
had not been seen by a nephrologist 1 year prior to the start of RRT, and 33
percent had not been seen even 3 months before RRT.[53]
Although control of diet is a major aspect of care for patients with chronic
kidney failure and terminal kidney failure, by the start of RRT, 46 percent of
the patients had not seen a dietitian.
Target: 50
percent.
Baseline: 29
percentof newly diagnosed patients
with treated chronic kidney failure on hemodialysis used arteriovenous fistulas
as the primary mode of
vascular access in 1997.
Target setting
method: 72 percent improvement (consistent with Dialysis
outcomes quality initiative [doqi] guidelines). (better than the best will be
used when data are available.)
Data source:
U.S. Renal Data System (USRDS), NIH, NIDDK.
|
Newly Diagnosed Patients
With Treated Chronic Kidney Failure on Hemodialysis, 1997
|
Arteriovenous
Fistula Use
|
|
Percent
|
|
TOTAL
|
29
|
|
Race and ethnicity
|
|
American Indian or Alaska Native
|
DNA
|
|
Asian or Pacific Islander
|
DNA
|
|
Asian
|
DNC
|
|
Native Hawaiian and other
Pacific
Islander
|
DNC
|
|
Black or African American
|
DNA
|
|
White
|
DNA
|
|
|
|
Hispanic or Latino
|
DNA
|
|
Not Hispanic or Latino
|
DNA
|
|
Black or African American
|
DNA
|
|
White
|
DNA
|
|
Gender
|
|
Female
|
DNA
|
|
Male
|
DNA
|
|
Family income level
|
|
Poor
|
DNC
|
|
Near Poor
|
DNC
|
|
Middle/high income
|
DNC
|
DNA = Data have not been analyzed. DNC = Data are not
collected. DSU = Data are statistically unreliable.
Patients receiving renal
replacement therapy as of December 31, 1997, were treated predominantly (72
percent) with dialysis. Of these, 88 percent were on hemodialysis. Vascular
access is the major lifeline for hemodialysis patients. The presence of a
functioning vascular access site represents a critical factor in the well-being
of these patients. Unfortunately, however, it also is the largest single cause
of illness and disability in patients receiving hemodialysis for renal replacement
therapy, accounting for nearly 25 percent of all hospitalizations. Complications
and problems related to vascular access have been estimated to account for as
much as 17 percent of the health care costs associated with treated chronic
kidney failure.[54]
Monitoring the type of
vascular access for dialysis in new patients is an important method to assess
the adequacy of preparation for RRT. Clinical evidence shows that patients with
endogenous arteriovenous fistulas experience lower complication rates than
patients with synthetic grafts. In the United States, the use rate for
arteriovenous fistulas is under 30 percent.[55] Arteriovenous fistulas, ideally,
should be placed at least 6 months before the start of dialysis. Early
placement of arteriovenous fistulas is particularly important for elderly
persons, becauseatheroscleroticvessels may take a much longer
time to dilate to a usable diameter.
Target: 66
percent of dialysis patients.
Baseline: 20
percent of newly diagnosed treated chronic kidney failure patients under age 70
years were registered on the waiting list in 1994–96.
Target setting
method: Better than the best.
Data source:
U.S. Renal Data System (USRDS), NIH, NIDDK.
|
Dialysis Patients Under
Age 70 Years,
1994–96
|
Transplant
Waiting List
|
|
Percent
|
|
TOTAL
|
20
|
|
Race and ethnicity
|
|
American Indian or Alaska Native
|
2
|
|
Asian or Pacific Islander
|
4
|
|
Asian
|
DNC
|
|
Native Hawaiian and other
Pacific
Islander
|
DNC
|
|
Black or African American
|
29
|
|
White
|
65
|
|
|
|
Hispanic or Latino
|
12
|
|
Not Hispanic or Latino
|
DNA
|
|
Black or African American
|
DNA
|
|
White
|
DNA
|
|
Gender
|
|
Female
|
40
|
|
Male
|
60
|
|
Family income level
|
|
Poor
|
DNC
|
|
Near Poor
|
DNC
|
|
Middle/high income
|
DNC
|
|
Select populations
|
|
Age groups
|
|
Under
20 years
|
3
|
|
20
to 39 years
|
31
|
|
40 to 59 years
|
51
|
|
60 to 69 years
|
15
|
DNA = Data have not been analyzed.
DNC = Data are not collected. DSU = Data are statistically unreliable.
Successful renal
transplantation confers many advantages, including improvements in physical and
psychological growth in children and improved survival and quality of life for
recipients in general. The prospects of receiving a kidney transplant, however,
are determined by a number of factors. These factors include age, primary cause
of kidney failure, race and ethnic origin, gender, geographic location, and
availability of suitable donors. Any combination of these factors may directly
influence the first important step in the process of receiving a kidney
transplant—namely, being registered on the waiting list. Significant
disparities exist in the people who are registered on the waiting list. Women
and people from certain racial and ethnic groups—particularly, African
Americans—are less likely than other kidney transplant candidates to be
registered on the waiting list.40, 55, [56]
Target: 51
registrants per 1,000 patient years at risk.
Baseline: 41
registrants per 1,000 patient years at risk (since placed on dialysis) received
a transplant within 3 years in 1995–97.
Target setting
method: Better than the best.
Data source: U.S.
Renal Data System (USRDS), NIH, NIDDK.
Renal Transplant Waiting
List Registrants, 1995–97
|
Transplant
Within 3 Years
|
|
Rate per 1,000
Patient Years
|
|
TOTAL
|
41
|
|
Race and ethnicity
|
|
American Indian or Alaska Native
|
30
|
|
Asian or Pacific Islander
|
DNA
|
|
Asian
|
50
|
|
Native Hawaiian and other
Pacific
Islander
|
DNA
|
|
Black or African American
|
30
|
|
White
|
49
|
|
|
|
Hispanic or Latino
|
DNA
|
|
Not
Hispanic or Latino
|
DNA
|
|
Black
or African American
|
DNA
|
|
White
|
DNA
|
|
Gender
|
|
Female
|
33
|
|
Male
|
49
|
|
Family income level
|
|
Poor
|
DNC
|
|
Near Poor
|
DNC
|
|
Middle/high income
|
DNC
|
|
Select populations
|
|
Age groups
|
|
Under 20 years
|
282
|
|
20 to 44 years
|
110
|
|
45
to 64 years
|
52
|
|
65
years and older
|
6
|
DNA = Data have not been analyzed. DNC = Data are not
collected. DSU = Data are statistically unreliable.
Individuals from certain
racial and ethnic populations (specifically, African Americans) move up the
waiting list to receive kidney transplants at a slower rate than whites.[57], [58]
The exact causes are unclear. Racial and ethnic disparities in waiting times
may be influenced by genetic and biological factors (such as HLA types),[59]
the request and consent procedures of organ procurement organizations, patient
registration practices for a center or region, organ acceptance practices at
each transplant center, geographic location, socioeconomic status, cultural attitudes
and beliefs about organ donation, rates of organ donation within each local
area, and the donor pool.42, [60]
Reports also have documented
a lower rate of transplantation in women.55
The U.S. Department of Health and Human Services (HHS) is working toward the
goal of making sure that all persons in the United States have an equal opportunity
to receive a transplant, regardless of who they are or where they live. To increase
access to transplantation, HHS launched the National Organ and Tissue Donation
Initiative to increase overall organ and tissue donation. One aspect of the
initiative is to learn more about the factors that influence organ and tissue donation,
with a special emphasis on certain racial and ethnic communities. Health care
workers, particularly in the area of transplantation, need to understand the
various obstacles to organ donation and transplantation, especially in the
groups with which they work, and to initiate programs and policies that are
culturally sensitive and meaningful.
Target: 78
diabetic persons with new cases of ESRD per million population.
Baseline: 113
diabetic persons with new cases of ESRD per million population were reported in
1996.
Target setting
method: Better than the best.
Data source: U.S.
Renal Data System (USRDS), NIH, NIDDK.
|
Persons With Diabetes,
1996
|
New Cases of ESRD
|
|
Rate per Million
|
|
TOTAL
|
113
|
|
Race and ethnicity
|
|
American Indian or Alaska Native
|
482
|
|
Asian or Pacific Islander
|
156
|
|
Asian
|
DNC
|
|
Native Hawaiian and other Pacific Islander
|
DNC
|
|
Black or African American
|
329
|
|
White
|
79
|
|
|
|
Hispanic or Latino
|
DNA
|
|
Not Hispanic or Latino
|
DNA
|
|
Black or African American
|
DNA
|
|
White
|
DNA
|
|
Gender
|
|
Female
|
103
|
|
Male
|
112
|
|
Family income level
|
|
Poor
|
DNC
|
|
Near Poor
|
DNC
|
|
Middle/high income
|
DNC
|
|
Select populations
|
|
Age groups
|
|
Under
20 years
|
0
|
|
20
to 44 years
|
35
|
|
45
to 64 years
|
276
|
|
65 to 74 years
|
514
|
|
75 years and older
|
263
|
DNA = Data have not been analyzed. DNC = Data are not
collected. DSU = Data are statistically unreliable.
Convincing, consistent, and
continuing scientific evidence shows that with secondary and tertiary
prevention, microvascular complications of diabetes, especially diabetic kidney
disease, can be reduced substantially. Enhanced quality of life, reductions in
death rates, and reduced costs can result from improved clinical and public
health diabetes prevention strategies directed at kidney disease and other
microvascular and metabolic complications of diabetes. Monitoring the consequences
of these strategies, including reductions in the magnitude of chronic renal
insufficiency, terminal kidney failure, and other microvascular complications,
should be an important component of an effective national public health program.
Potential data sources: National Ambulatory
Medical Care Survey (NAMCS), CDC, NCHS; National Hospital Ambulatory Medical
Care Survey (NHAMCS), CDC, NCHS.
 |  |
1. |
Access to
Quality Health Services |
 |  |
5. |
Diabetes |
 |
 |
 |
 |
 | 5-2. |  | New cases of
diabetes |
 | 5-3. |  | Overall cases of
diagnosed diabetes |
 | 5-4. |  | Diagnosis of
diabetes |
 | 5-7. |  | Cardiovascular
disease deaths in persons with diabetes |
 | 5-11. |  | Annual urinary
microalbumin measurement |
 | 5-12. |  | Annual
glycosylated hemoglobin measurement |
 |  |
6. |
Disability
and Secondary Conditions |
 |
 |
 |
 |
 | 6-1. |  | Standard
definition of people with disabilities in data sets |
 | 6-2. |  | Feelings and
depression among children with disabilities |
 | 6-3. |  | Feelings and depression interfering with
activities among adults with disabilities |
 | 6-5. |  | Sufficient
emotional support among adults with disabilities |
 | 6-6. |  | Satisfaction
with life among adults with disabilities |
 | 6-8. |  | Employment
parity |
 |  |
7. |
Educational
and Community-Based Programs |
 |
 |
 |
 |
 | 7-7. |  | Patient and
family education |
 | 7-8. |  | Satisfaction
with patient education |
 | 7-9. |  | Health care organization sponsorship of
community health promotion activities |
 | 7-10. |  | Community health
promotion programs |
 | 7-11. |  | Culturally appropriate and linguistically
competent community health promotion programs |
 |  |
8. |
Environmental
Health |
 |
 |
 |
 |
 | 8-11. |  | Elevated blood
lead levels in children |
 | 8-14. |  | Toxic pollutants |
 | 8-20. |  | School policies
to protect against environmental hazards |
 | 8-22. |  | Lead-based paint
testing |
 | 8-25. |  | Exposure to heavy
metals and other toxic chemicals |
 | 8-26. |  | Information
systems used for environmental health |
 | 8-27. |  | Monitoring
environmentally related diseases |
 | 8-29. |  | Global burden of
disease |
 |  |
10. |
Food Safety |
 |  |
11. |
Health
Communication |
 |  |
12. | Heart Disease and Stroke |
 |
 |
 |
 |
 | 12-8. |  | Knowledge of
early warning symptoms of stroke |
 | 12-9. |  | High blood
pressure |
 | 12-10. |  | High blood
pressure control |
 | 12-11. |  | Action to help
control blood pressure |
 | 12-12. |  | Blood pressure
monitoring |
 | 12-16. |  | LDL-cholesterol
level in CHD patients |
 |  |
13. |
HIV |
 |
 |
 |
 |
 | 13-1. |  | New AIDS cases |
 | 13-3. |  | AIDS among
persons who inject drugs |
 | 13-5. |  | New HIV cases |
 | 13-8. |  | HIV counseling
and education for persons in substance abuse treatment |
 | 13-12. |  | Screening for STDs
and immunization for hepatitis B |
 | 13-17. |  | Perinatally
acquired HIV infection |
 |  |
14. |
Immunization
and Infectious Diseases |
 |
 |
 |
 |
 | 14-1. |  | Vaccine-preventable
diseases |
 | 14-2. |  | Hepatitis B in
infants and young children |
 | 14-3. |  | Hepatitis B in
adults and high-risk groups |
 | 14-9. |  | Hepatitis C |
 | 14-10. |  | Identification of
persons with chronic hepatitis C |
 | 14-16. |  | Invasive early
onset group B streptococcal disease |
 | 14-28. |  | Hepatitis B
vaccination among high-risk groups |
 |  |
16. |
Maternal,
Infant, and Child Health |
 |  |
17. |
Medical
Product Safety |
 |  |
19. |
Nutrition and
Overweight |
 |  |
20. |
Occupational
Safety and Health |
 |  |
22. |
Physical
Activity and Fitness |
 |  |
23. |
Public Health
Infrastructure |
 |
 |
 |
 |
 | 23-2. |  | Public access to
information and surveillance data |
 | 23-3. |  | Use of geocoding
in health data systems |
 | 23-4. |  | Data for all
population groups |
 | 23-5. |  | Data for Leading Health Indicators, Health
Status Indicators, and Priority Data Needs at State, Tribal, and local levels |
 | 23-6. |  | National tracking
of Healthy People 2010 objectives |
 | 23-7. |  | Timely release of
data on objectives |
 | 23-17. |  | Population-based
prevention research |
 |  |
25. |
Sexually
Transmitted Diseases |
 |  |
27. |
Tobacco Use |
 |
 |
 |
 |
 | 27-1. |  | Adult tobacco use |
 | 27-2. |  | Adolescent
tobacco use |
 | 27-5. |  | Smoking cessation
by adults |
 | 27-7. |  | Smoking cessation
by adolescents |
 | 27-10. |  | Exposure to environmental tobacco smoke |
(A listing of abbreviations and acronyms used in this
publication appears in Appendix H.)
Arteriovenous
fistula: The type of vascular access created by joining a person’s own
(endogenous) artery to the nearby vein. The increase in blood flow in the vein
leads to marked dilation of the vein and permits an easier insertion of needles
for dialysis.
Chronic renal insufficiency, chronic renal failure, and
end-stage renal disease (ESRD) (defined in this chapter as treated
chronic kidney failure): Terms describing the continuum of increasing renal
dysfunction and decreasing glomerular filtration rate (GFR). Because of the
progressive nature of kidney disease, these terms represent successive stages
of disease in most patients.
Chronic renal insufficiency: The stage in chronic kidney disease in
which damage to the kidney already has resulted in significant impairment of
renal function, but systemic manifestations are minimal. Most patients who have
chronic renal insufficiency are asymptomatic. Chronic renal insufficiency
usually is identified because the serum creatinine is slightly elevated
(greater than 1.5 mg/dL in males or 1.2 mg/dL in females and greater than age-specific
normative values in children). The serum creatinine test is insensitive and
does not identify all persons who have chronic renal insufficiency. Although
precise GFR limits cannot be assigned to this stage of disease, typically
patients with chronic renal insufficiency have a GFR between 30 ml/min and 75
ml/min.
Chronic renal failure: The stage in chronic renal disease in which
renal dysfunction has progressed to a level resulting in systemic
manifestations. These manifestations include a rise in the blood concentration
of urea, creatinine, and phosphate, which are removed by the kidneys, and other
problems, such as anemia, bone disease, acidosis, and salt and fluid retention.
Growth failure may be seen in children. Most patients with chronic renal
failure progress to treated chronic kidney failure (end-stage renal disease).
End-stage renal disease (ESRD) (referred to in this focus area as
treated chronic kidney failure): The stage in chronic renal disease in
which renal replacement therapy, dialysis, or kidney transplantation is needed
to sustain life. Treated chronic kidney failure is generally an irreversible
state. The glomerular filtration rate is usually less than 10 ml/min.
Diabetes (diabetes
mellitus): A chronic disease due to insulin deficiency or resistance to
insulin action and associated with hyperglycemia (elevated blood glucose
levels). Over time, without proper preventive treatment, organ complications
related to diabetes develop, including heart, nerve, foot, eye, and kidney
damage as well as problems with pregnancy. Diabetes is classified into two major
categories.
Type 1 diabetes (previously
called insulin-dependent diabetes mellitus [IDDM] or juvenile-onset
diabetes [JODM]): Represents clinically about 5 percent of all persons with
diagnosed diabetes. Its clinical onset typically occurs at ages under 30 years,
with more gradual development after age 30. Most often type 1 diabetes
represents an autoimmune destructive disease in the beta (insulin-producing)
cells of the pancreas in genetically susceptible individuals. Insulin therapy
always is required to sustain life and maintain diabetes control.
Type 2 diabetes
(previously called noninsulin-dependent diabetes mellitus [NIDDM] or
adult-onset diabetes [AODM]): Refers to the most common form of diabetes
in the United States and the world, especially in certain racial and ethnic
groups and in elderly persons. Women who develop diabetes during pregnancy also
are at increased risk of developing this type of diabetes later in life. In the
United States, approximately 95 percent of all persons with diagnosed diabetes
(10.5 million) and 100 percent of all persons with undiagnosed (5.5 million)
diabetes probably have type 2 diabetes.
Diabetic kidney
disease: Kidney disease and resultant kidney functional impairment due to
the longstanding effects of diabetes on the microvasculature of the kidney.
Features include increased urine protein and decreased kidney function.
Dialysis: The process by which metabolic waste
products are removed by cleansing of the blood directly through extracorporeal
filtration membranes (hemodialysis) or indirectly by diffusion of waste
products through the peritoneal membranes into instilled fluids (peritoneal
dialysis).
End-stage renal
disease: See above.
Glomerular
filtration: The process by which the kidneys filter the blood, clearing it
of toxins.
Glomerular filtration
rate (GFR): The rate at which the blood is cleared by glomerular filtration
and an important measure of kidney function. Normal GFR values in adults are
between 100 and 150 ml/min. One of the most important hallmarks of chronic
renal disease is a progressive decline in the rate of glomerular filtration.
Generally, a GFR below 75 ml/min represents clinically significant renal
insufficiency. A GFR of less than 10 ml/min represents kidney failure severe
enough to require renal replacement therapy to maintain life.
Hemodialysis: The
process by which biologic waste products are removed from the body through
external blood circuit and external (artificial) membranes.
Glomerulonephritis: Inflammation
in the primary filtration units (glomeruli) of the kidneys. Typically, this
process leads to loss of blood, blood products, and protein into the urine.
Unchecked or without effective treatment, this process could lead ultimately to
permanent kidney damage and loss of kidney function and chronic kidney failure.
Incidence rate: A
measure of the number of new cases of disease occurring in a specific
population over a specific period of time, usually 1 year. For end-stage
renal disease, the best information is based on the incidence of treated end-stage
kidney disease reported through Medicare to the U.S. Renal Data System. Available
data exclude those patients who die without receiving treatment.
Interstitial
nephritis: Inflammation in the supporting matrix of the kidneys. This
process could result from damage caused by microorganisms (such as bacteria and
viruses) or from toxic reaction to drugs or other substances such as lead and
mercury.
Microalbuminuria: Abnormally
elevated levels of albumin in the urine—but at levels too low to be detectable
by the dipstick method used to test for protein in the urine. Increased urinary
excretion of albumin, even if the concentration is too low to be detectable as
dipstick proteinuria, has been associated with increased risk of progressive
kidney disease in people with diabetes6, 7and increased risk of subsequent death in persons with8 and without diabetes9,
10 and in elderly individuals.11
Microalbuminuria can be measured in several ways. If a random urine sample is
used, the albumin concentration in the first-voided morning urine or the
ratio of urine albumin to urine creatinine can be used. If a timed urine
collection is available, an albumin excretion rate can be determined. Urine
albumin concentrations of 30 to 300 mg/ml, urinary albumin to
creatinine ratios of less than 3.5 mg/mmol, and urine albumin excretion rates of less than 15 mg/min all have been used
as cutoff values for detection of microalbuminuria.
Patient year (at
risk): A measure of the duration (in years) a patient has been exposed to
the effects of a particular biologic or physiologic condition, such as chronic
renal insufficiency or the effects of dialysis.
Polycystic kidney disease:
A disorder (usually inherited) of the kidneys in which the normal kidney
structures (particularly, the tubules) are replaced by sacs (or cysts) that
ultimately increase in size and lead to further destruction of the supporting
matrix of the kidneys. The most common variety is the adult polycystic kidney
disease (ADPKD), which is inherited as an autosomal dominant genetic disease.
ADPKD is usually characterized by elevated blood pressure, pain from enlarged
cysts, blood in the urine, and a relentless progression to terminal kidney
failure.
Prevalence rate: A
measure of the total number of cases of disease existing in a specific
population at a certain point in time (point prevalence) or over a certain period
of time (period prevalence). Point-prevalence rates reflect the number of individuals
at the stated date.
Proteinuria: Abnormal
levels of protein in the urine. Proteinuria is a marker for structural kidney
damage or inflammation and also may be involved in the pathogenesis of progressive
renal injury. Increased risks of developing progressive renal disease,1, 2 of death,3, 4
and of death due to cardiovascular disease5
have been documented in persons with persistent proteinuria. Urine protein can
be estimated by a dipstick method, which provides a semiquantitative estimate
of concentration. More accurate measures include determining the ratio of urine
protein to urine creatinine or the amount of protein excreted by a person in a
24-hour period.
Renal disease: A
synonym for kidney disease.
Serum creatinine: A
blood chemistry measurement used to estimate the level of kidney function.
Serum creatinine is an important index for monitoring progression of disease in
persons with chronic renal disease. Elevations in serum creatinine are an
insensitive marker of early chronic renal insufficiency. In advanced renal
failure, however, a change in serum creatinine is a more reliable indicator.
This test remains the most widely available method used to estimate the
glomerular filtration rate or to monitor changes in level of renal function.12
U.S. Renal Data
System (USRDS): A national database of information on treated chronic
kidney failure patients—new cases, illness, disability, and death outcomes.
USRDS is based predominantly on data collected by the Health Care Financing
Administration’s Medicare treated chronic kidney failure program and is funded
by a contract from the National Institutes of Health.13 This
database contains information on approximately 93 percent of all patients
treated for treated chronic kidney failure in the United States. Most of the
data cited in this focus area derive from USRDS reports. As noted, these
numbers reflect reported cases of treated end-stage renal disease and,
therefore, do not include patients who die without treatment or patients whose
care is not reported to USRDS.
Vascular access: The
means by which blood is removed from a person for cleansing during dialysis and
safely and easily returned, when cleaned, into the body.
Vasculitis: Inflammation
of the blood vessels. Typically, the cause of this process is unknown.
Untreated, it leads to progression to relentless and specific organ failures,
including chronic kidney failure, or death.
[1] Jones, C.A.; McQuillan, G.M.; Kusek,
J.W.; et al. Serum creatinine levels in the U.S. population: Third
National Health and Nutrition Examination Survey. American Journal of Kidney Diseases 32:992-999, 1998.
PubMed; PMID 9856515
[2]
U.S. Renal Data System (USRDS). 1999 Annual
Data Report (ADR). Bethesda, MD: National Institutes of Health (NIH),
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), April
1999, Appendix, Table A-1.
[3]
USRDS. 1999 ADR. Bethesda, MD:
NIH, NIDDK, April 1999, Appendix, Tables A-1 and B-3.
[4]
USRDS. 1999 ADR. Bethesda, MD:
NIH, NIDDK, April 1999, Chapter X, 133-148.
[5]
USRDS. 1999 ADR. Bethesda, MD:
NIH, NIDDK, April 1999, Chapter V, 63-78.
[6]
USRDS. 1999 ADR. Bethesda, MD:
NIH, NIDDK, April 1999, Appendix, Table A-1.
[7]
USRDS. 1999 ADR. Bethesda, MD:
NIH, NIDDK, April 1999, Appendix, Table A-15.
[8]
USRDS. 1999 ADR. Bethesda, MD:
NIH, NIDDK, April 1999, Appendix, Table A-3.
[9]
USRDS. 1999 ADR. Bethesda, MD: NIH, NIDDK, April 1999, Appendix, Table A-1.
[10]
National Heart, Lung, and Blood Institute. Morbidity
and Mortality Chartbook on Cardiovascular Disease, Lung and Blood Diseases. Bethesda,
MD: Public Health Service, 1998.
[11]
USRDS. 1999 ADR. Bethesda, MD:
NIH, NIDDK, April 1999, Appendix, Tables A-1, A-2, and A-3.
[12]
Clark, C. How should we respond to the worldwide diabetes epidemic? Diabetes Care 21:475-476, 1998.
PubMed; PMID 9571326
[13]
Centers for Disease Control and Prevention (CDC). National Diabetes Fact Sheet: National Estimates and General Information
on Diabetes in the United States. Atlanta, GA: U.S. Department of Health
and Human Services, 1997.
[14]
USRDS. 1999 ADR. Bethesda, MD:
NIH, NIDDK, April 1999, Appendix, Table A-6.
[15]
Eggers, P.W. Personal communication. Health Care Financing Administration.
1999.
[16]
Perneger, T.V.; Klag, M.J.; Feldman, H.I.; et al. Projection of hypertension
related renal disease in middle-aged residents of the United States. Journal of the American Medical Association 269(10):1272-1277,
1993.
PubMed; PMID 8437305
[17]
Whittle, J.C.; Whelton, P.K.; Seidler, A.J.; et al. Does racial variation in
risk factors explain black-white differences in the incidence of hypertensive
end-stage renal disease? Archives
of Internal Medicine 151:1359-1364, 1991.
PubMed; PMID 2064486
[18]
Brancati, F.L.; Whittle, J.C.; Whelton, P.K.; et al. The excess incidence of
diabetic end-stage renal disease among blacks. A population based study of
potential explanatory factors. Journal of
the American Medical Association 268:3079-3084, 1992.
PubMed; PMID 1433738
[19]
USRDS. 1999 ADR. Bethesda, MD:
NIH, NIDDK, April 1999, Appendix, Tables A-1 and A-14.
[20]
Gonzales Villalpano, C.G.; Stern, M.P.; Arrendondo Perez, B.; et al.
Nephropathy in low income diabetics: The Mexico City Diabetes Study. Archives of Medical Research 27:367-372,
1996.
[21]
Pugh, J.A.; Stern, M.P.; Haffner, S.M.; et al. Excess incidence of treatment of
end-stage renal disease in Mexican Americans. American Journal of Epidemiology 127:135-144, 1998.
PubMed; PMID 3276155
[22]
Agodoa, L.Y.C.; Roys, E.C.; Wolfe, R.A.; et al. ESRD among Hispanics in the
U.S. Journal of the American Society of Nephrology
10:232A, 1999.
[23]
USRDS. 1999 ADR. Bethesda, MD:
NIH, NIDDK, April 1999, Appendix, Table B-3.
[24]
USRDS. 1999 ADR. Bethesda, MD:
NIH, NIDDK, April 1999, Appendix, Table ES-1.
[25]
USRDS. 1999 ADR. Bethesda, MD:
NIH, NIDDK, April 1999, Appendix, Table A-6.
[26]
Rettig, R., and Levinsky, N.G. Kidney Failure and the Federal Government.
Washington, DC: National Academy Press, 1991.
[27] Port, F.K.; Wolfe, B.A.; Mauger, E.A.; et
al. Comparison of survival probabilities for dialysis patients vs.
cadaveric renal transplant recipients. Journal of the American Medical
Association 270(11):1339-1343, 1993.
PubMed; PMID 8360969
[28]
USRDS. 1999 ADR. Bethesda, MD: NIH, NIDDK, April 1999, Appendix, Table
F-1.
[29]
USRDS. 1999 ADR. Bethesda, MD: NIH, NIDDK, April 1999, Chapter VII,
104-106.
[30]
USRDS. 1999 ADR. Bethesda, MD: NIH, NIDDK, April 1999, Appendix, Table
F-2.
[31]
Freedman, B.I.; Tuttle, A.B.; and Spray, B.J. Familial predisposition to nephropathy
in African Americans with non-insulin-dependent diabetes mellitus. Kidney Diseases 25(5):710-713, 1995.
PubMed; PMID 7747724
[32]
Siegel, J.; Krolewski, A.; Warran, J.; et al. Cost-effectiveness of screening
and early treatment of nephropathy in patients with insulin-dependent diabetes
mellitus. Journal of the American Society
of Nephrology 3:S111-S119,
1992.
PubMed; PMID 1457753
[33]
Kiberd, B., and Jindal, K. Screening to prevent renal failure in insulin
dependent diabetic patients: An economic evaluation. British Medical Journal 311:1595-1599, 1995.
PubMed; PMID 8555801
[34]
Borch-Johnsen, K.; Wenzel, H.; Viberti, G.; et al. Is screening and
intervention for microalbuminuria worthwhile in patients with insulin dependent
diabetes? British Medical Journal 306:1722-1725,
1993.
PubMed; PMID 8343628
[35]
Ifudu, O.; Dawood, M.; Homel, P.; et al. Excess morbidity in patients starting
uremia therapy without prior care by a nephrologist. American Journal of Kidney Diseases 28:841-845, 1996.
PubMed; PMID 8957035
[36]
USRDS. 1997 ADR. Bethesda, MD: NIH,
NIDDK, April 1997, 49-60.
[37]
USRDS. 1998 ADR. Bethesda, MD: NIH, NIDDK,April
1998, Table G-19.
[38]
USRDS. 1998 ADR. Bethesda, MD: NIH,
NIDDK, April 1998, Appendix, Table G-43.
[39] Turenne, M.N.; Port, F.K.; Strawderman,
R.L.; et al. Growth rates in pediatric dialysis patients and renal
transplant recipients. American Journal
of Kidney Diseases 30(2):193-203, 1997.
PubMed; PMID 9261029
[40]
United Network for Organ Sharing (UNOS). 1997
Report of the Organ Procurement and Transplantation Network: Waiting List
Activity and Donor Procurement. Executive Summary, Kidney Volume. Richmond, VA: UNOS, 1997.
[41]
Alexander, G.C., and Sehgal, A.R. Barriers to cadaveric renal transplantation
among blacks, women, and the poor. Journal
of the American Medical Association 280(13):1148-1152, 1998.
PubMed; PMID 9777814
[42]
Bloembergen, W.E.; Mauger, E.A.; and Wolfe, R.A. Association of gender and
access to cadaveric renal transplantation. American
Journal of Kidney Diseases 30(6):733-738, 1997.
PubMed; PMID 9398115
[43] Narva, A.; Stiles, S.; Karp, S.; et al. Access
of Native Americans to renal transplantation in Arizona and New Mexico. Blood Purification 14:293-304,
1996.
PubMed; PMID 8873955
[44]
Ozminkowski, R.J.; White, A.J.; Hassol, A.; et al. Minimizing racial disparity
regarding receipt of a cadaver kidney transplant. American Journal of Kidney Diseases 30:749-759, 1997.
PubMed; PMID 9398117
[45]
USRDS. 1997 ADR. Bethesda, MD: NIH,
NIDDK, April 1997, 107-112.
[46]
Levey, A.S.; Beto, J.A.; Coronado, B.E.; et al. Controlling the epidemic of
cardiovascular disease in chronic renal disease. What do we know? What do we
need to learn? Where do we go from here? Report
of the National Kidney Foundation Task Force on Cardiovascular Disease. New
York, NY: National Kidney Foundation, July 1998.
[47]
Bostom, A.G.; Shemin, D.; Verhoef, P.; et al. Elevated fasting total plasma
homocysteine levels and cardiovascular disease outcomes in maintenance dialysis
patients: A prospective study. Arteriosclerosis,
Thrombosis, and Vascular Biology 17(11):2554-2558, 1997.
PubMed; PMID 9409227
[48]
Bostom, A.G., and Lathrop, L. Hyperhomocysteinemia in end-stage renal
disease: Prevalence, etiology, and potential relationship to arteriosclerotic
outcomes. Kidney International 52(1):10-20,
1997.
PubMed; PMID 9211341
[49]
Bostom, A.G.; Shemin, D.; Lapane, K.L.; et al. Hyperhomocysteinemia, hyperfibrinogenemia,
and lipoprotein (a) excess in maintenance dialysis patients: A matched
case-control study. Arteriosclerosis 125(1):91-101,
1996.
PubMed; PMID 8831931
[50]
Perneger, T.V.; Klag, M.J.; and Whelton, P.K. Cause of death in patients with
end-stage renal disease: Death certificates vs. registry reports. American Journal of Public Health 83(12):1735-1738,
1993.
PubMed; PMID 8259805
[51]
USRDS. 1997 ADR. Bethesda, MD:
NIH, NIDDK, April 1997, Figure IV-3, 52.
[52]
National Kidney Foundation. Dialysis Outcomes, Quality Initiatives, Clinical
Practice Guidelines. Executive Summaries. New York, NY: the Foundation,
1997.
[53]
USRDS. 1999 ADR. Bethesda, MD:
NIH, NIDDK, April 1999, 159.
[54] Hirth, R.A.; Turenne, M.N.; Woods, J.D.;
et al. Vascular access in newly treated U.S. hemodialysis patients:
Predictors and trends. Journal of the
American Medical Association 276:1303-1308, 1996.
PubMed; PMID 8861988
[55]
Soucie, J.M.; Neylan, J.F.; and McClellan, W. Race and sex differences in the
identification of candidates for renal transplantation. American Journal of Kidney Diseases 19:414-419, 1992.
PubMed; PMID 1585927
[56]
Kasiske, B.L.; London, W.; and Ellison, M.D. Race and socioeconomic factors
influencing early placement on the kidney transplant waiting list. Journal of the American Society of Nephrology
9:2142-2147, 1998.
PubMed; PMID 9808103
[57] Ellison, M.D.; Breen, T.J.; Guo, T.G.; et
al. Blacks and whites on the UNOS renal waiting list: Waiting times and
patient demographics compared. Transplantation
Proceedings 25(4):2462-2466, 1993.
PubMed; PMID 8356634
[58]
Thompson, J.S. American Society of Histocompatibility and Immunogenetics CrossMatch
Study. Transplantation 59(11):1636-1638,
1995.
PubMed; PMID 7778183
[59] Sanfilippo, F.P.; Vaughn, W.K.; Peters,
T.G.; et al. Factors affecting the waiting time of cadaveric kidney
transplant candidates in the United States. Journal
of the American Medical Association 267:247-252, 1992.
PubMed; PMID 1727521
[60]
McCauley, J.; Irish, W.; Thompson, L.; et al. Factors determining the rate of
referral, transplantation, and survival on dialysis in women with ESRD. American Journal of Kidney Diseases 30:739-748, 1997.
PubMed; PMID 9398116


