This page is for historical and reference purposes only. Updates to content and links will not be maintained. Current information about NIDDK's Kidney, Urological and Hematology programs can be found at http://www2.niddk.nih.gov/Research/ScientificAreas/Kidney/, http://www2.niddk.nih.gov/Research/ScientificAreas/Urology/ and http://www2.niddk.nih.gov/Research/ScientificAreas/Hematology/.
The American Society of Nephology will meet at the Colorado Convention Center from November 16 to November 21, 2010.
Meeting Posters (PDF, 1.6 MB) Printer friendly version of the contents on this page
Funding History and Research Opportunities
NIDDK Funding History and Opportunities
The National Institutes of Health (NIH) comprises 27 separate Institutes and Centers and is the largest biomedical research center in the world. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) was established by Congress in 1950. Within the NIDDK, the Division of Kidney, Urology and Hematology (KUH) manages programs in kidney research.
In 2009, NIDDK awarded >$250M for kidney research.
Exploratory Research (R21) Program
The evolution and vitality of biomedical science requires constant infusion of new ideas, techniques, and points of view. These innovations may differ substantially from current thinking or practice, and may not yet be supported by substantial evidence. The NIDDK R21 program provides a mechanism dedicated to:
- Innovative, high pay-off, paradigm-shifting projects
- Novel technology and tool development
- Applications of existing methods, technologies, or conceptual approaches from outside biomedical science to a problem in the NIDDK mission
- Pilot clinical trials or clinical studies
The following projects are NOT suitable for the R21 mechanism:
- Projects of limited scope or cost that use widely accepted approaches and methods within established fields are NOT appropriate.
- A proposal designed to generate preliminary data for a longer-term project in a well-established research area is NOT appropriate.
- Projects by new PIs to gather preliminary data for an R01 are NOT appropriate and will NOT be given special priority for funding.
Most R21 applications can be submitted in response to:
- NIH Exploratory Research Grant Program (NIH Parent R21) PA-06-18
- Pilot & Feasibility Clinical Research Grants in Kidney Diseases (R21) PAR-06-113
- Secondary Analyses in Obesity, Diabetes, Digestive & Kidney Diseases (R21) PA-06-151
Upcoming NIDDK Meetings
- Clinical Trials in Acute Kidney Injury: Current Opportunities & Barriers
December 2, 2010, Bethesda, MD - Life After K: Completing the Transition to Independence
April 18-19, 2011, Washington, DC - Mechanisms of Organ Repair and Regeneration
September 14-16, 2011, Washington, DC
Collaborative Research
Complex biomedical science often requires the expertise of collaborating investigators working together as an investigative team. Collaborative research can be supported by several different types of grant mechanisms:
- R01 with a Principal Investigator and one or more key personnel or collaborators;
- Multi-PI R01 with multiple Principal Investigators collaborating and sharing credit and responsibility; or
- Center (P20/P30/P50) supporting a focused set of core activities.
- Program Project Grant (P01) designed to support a broadly based interrelated research program that has multiple distinct but synergistic research projects built around a unifying central theme; or
NEW!
- Resource-Related Research Project Grant (R24) designed to provide flexible support for an interdisciplinary research team focused on answering a single critically important research question. NIDDK is now accepting R24 applications which address research relevant to kidney, urologic and hematologic diseases
- Seeding Collaborative Interdisciplinary Team Science (R24) PAR-08-181
- Collaborative Interdisciplinary Team Science (R24) PAR-08-182
Animal Models of Diabetic Complications Consortium
An interdisciplinary consortium developing new animal models that closely mimic the human complications of diabetes for the purpose of studying disease pathogenesis, prevention and treatment. Consists of thirteen "pathobiology sites" studying diabetic nephropathy, uropathy, neuropathy, cardiomyopathy and vascular disease. A yearly Pilot and Feasibility Program allows access to new investigators with new ideas. Full details at www.amdcc.org.
Genitourinary Development Molecular Atlas Project
GUDMAP is a public database funded by the NIH to provide the scientific and medical community with tools to facilitate research. The key features of this database are: a molecular atlas of gene expression for the developing organs of the GenitoUrinary (GU) tract; a high resolution molecular anatomy that highlights development of the murine GU system; tutorials describing GU organogenesis; and the rapid access to primary data via the GUDMAP database. Full details at www.gudmap.org.
NEW!
NIH Director’s Early Independence Awards (DP5)
GOOGLE “NIH DP5”
Selected Funding Announcements
Basic Research
- Grants for Research in Glomerular Diseases (R01) PA-10-113
- Calcium Oxalate Stone Diseases (R01) PA-09-213
- Advances in Polycystic Kidney Disease (R01) PA-09-202
- Basic and Clinical Studies of Congenital Urinary Tract Obstruction (R01) PA-09-226
Translational Research
- Grants for Research in Glomerular Diseases (R01) PA-10-113
- Translational Research for the Prevention and Control of Diabetes and Obesity (R01) PAR-09-176
- Development of Assays for High-Throughput Drug Screening (R01) PA-10-213
- Development and Validation of Disease Biomarkers (R01) PA-09-204
- Non-Invasive Methods for Diagnosis and Progression of Diabetes, Kidney, Urological, Hematological & Digestive Diseases & Hypertensive Disorders (R01) PA-09-181
- Planning Grants for Translational Research for the Prevention and Control of Diabetes and Obesity (R18) PAR-09-177
- Planning Grants for Translating CKD Research into Improved Clinical Outcomes (R34) RFA-DK-10-011
Clinical Research
- Ancillary Studies of Acute Kidney Injury, Chronic Kidney Disease, and End Stage Renal Disease Accessing Information from Clinical Trials, Epidemiological Studies, and Databases (R01) PA-09-196
- NIDDK Small Grants for Clinical Scientists to Promote Diversity in Health-Related Research (R03) PAR-09-223
- Ancillary Studies to Major Ongoing NIDDK Clinical Research Studies to Advance Areas of Scientific Interest within the Mission of NIDDK (R01) PAR-09-247
- NIDDK Multi-Center Clinical Study Implementation Planning Grants (U34) PAR-10-197
- NIDDK Multi-Center Clinical Study Cooperative Agreement (U01) PAR-08-058
- Health Disparities in NIDDK Diseases (R01) PA-09-262
- Multidisciplinary Research in Critical Care (R01) PA-07-233
NEW!
37 month Time Limit for NIH Re-submission Applications
GOOGLE “NIH 37 time limit”
Small Business
Small Business Funding Opportunities
Why Seek SBIR/STTR Funds?
- Over $1 billion are available across NIH
- They provide seed money for high-risk projects
- They promote and foster partnerships with collaborators - including academia!
- Intellectual property rights are normally retained by small business
- Funds are NOT A LOAN - no repayment!
- Large corporations look to small companies for initial development
Small Business Innovation Research (SBIR)
The SBIR program supports innovative research conducted by small businesses to develop products for commercialization. The PI must be employed by the small business, but a research institution may be involved.
http://www.zyn.com/sbir
http://grants.nih.gov/grants/oer.htm
Small Business Technology Transfer (STTR)
The STTR program supports innovative research for products that have the potential for commercialization. STTR projects must be conducted cooperatively by a small business and a research institution.
http://www.zyn.com/sbir
http://grants.nih.gov/grants/oer.htm
Select NIDDK-Supported Small Business Projects
- Predicting kidney stones in relatives of stone formers
- Measurement of GFR (See PHARMACOPHOTONICS, LLC, POSTER #F-PO1102)
- Computerized Clinical Decision Support (See Visonex Booth #16525)
- Probiotic Use in CKD (See KIBOW BIOTECH INC. Booth #735)
- Commercialization of embryonic kidney stem cell lines, products and kits
- Cell Treatment for Septic Shock (See INNOVATIVE BIOTHERAPIES: Poster # F-PO1097, Poster # F-PO1109, Free Communication SA-FC466)
- Test for Salt-Sensitivity in Essential Hypertension
- Prevention / Treatment of Diabetic Nephropathy
- Intravital kidney multiphoton microscopy assays of therapeutic agents
- Renal perfusion quantification
- Tolerance induction in transplant patients
- Tracking Transplant Centers Performance (See CULMINI INC. Poster # SA-PO3060)
Training and Career Development
Post-Doctoral Training
Ruth L. Kirschstein National Research Service Awards (NRSA) - Individual (F32)
These awards provide support for fellows who have received their M.D., Ph.D., or other doctoral-level degree. Fellows need to identify a mentor and plan a research project before applying for 1 to 3 years of funding.
http://grants.nih.gov/grants/guide/pa-files/PA-10-110.html - Institutional (T32)
In place at many major universities, these grants provide pre- and postdoctoral support to fellows at those institutions. To be appointed to a training grant, contact the director of the training program at your institution.
http://grants.nih.gov/grants/guide/pa-files/PA-10-036.html
Training & Career Development Timeline
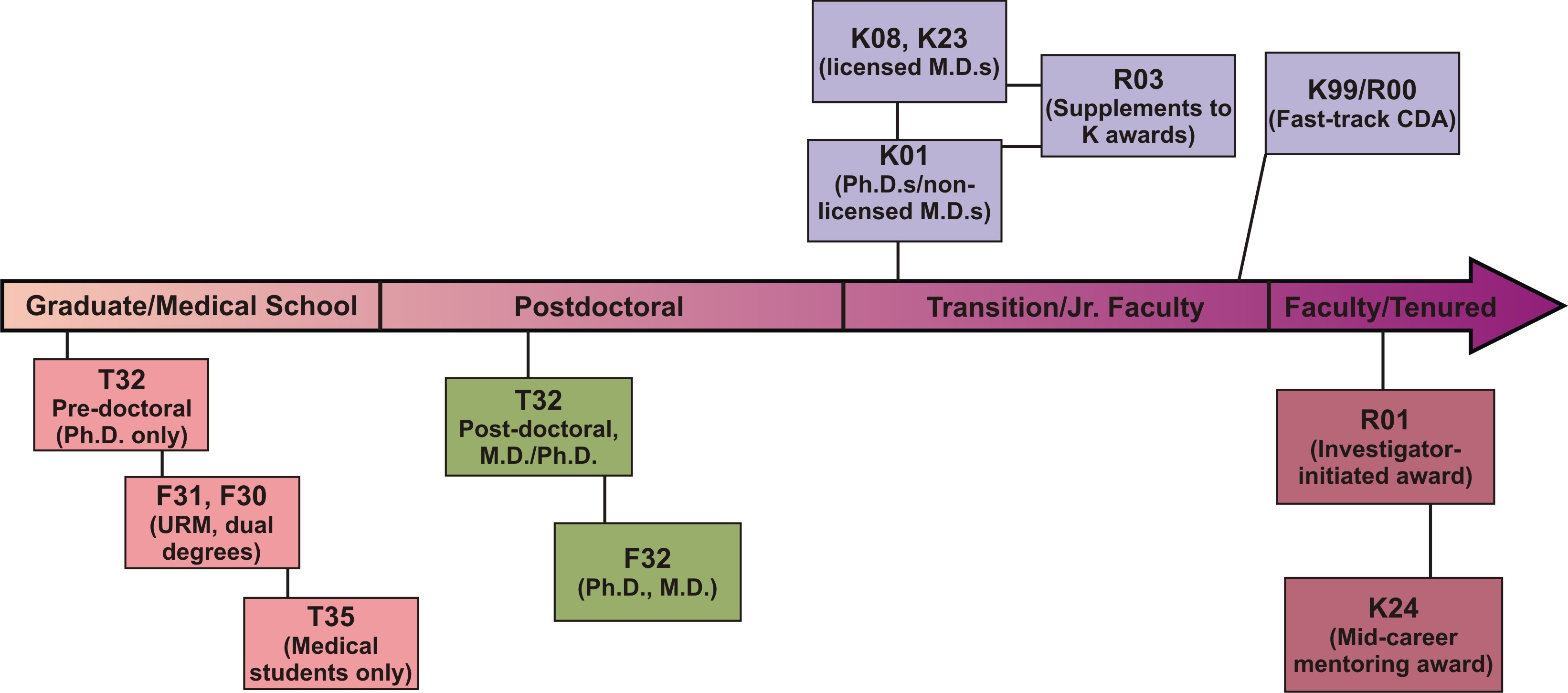
Loan Repayment Program
The goal of the Loan Repayment Program is to ease the debt burden clinical scientists may have incurred while attending medical school and a residency program. The NIDDK has two loan repayment programs: one for clinicians and one for pediatricians. In addition to these NIDDK programs, the NCMHD sponsors two other loan repayment programs for clinicians: one for those involved in health disparities research and another for clinical researchers from disadvantaged backgrounds. Competitive applicants must demonstrate their commitment to a research career and have a debt-to-salary ratio of at least 20 percent. The Loan Repayment Program may repay up to $35,000 a year toward each participant’s outstanding eligible educational loan debt, depending on total eligible repayable debt. For more details about eligibility and to apply online, visit http://www.lrp.nih.gov.
Career Development Awards*
- K01 (Mentored Research Scientist Development Awards)
Support Ph.D. scientists who have at least 3 to 5 years of postdoctoral training and who need to transition to independence. - K08 (Mentored Clinical Scientist Development Awards)
Aimed at physician-scientists to transition them to independence. - K23 (Mentored Patient-Oriented Research Career Development Awards)
Aimed at clinical investigators engaged in patient-based research. - K24 (Investigator Awards in Patient-Oriented Research)
Support mid-career physicians in patient-oriented research with funded clinical investigations and who are mentoring young clinicians. - K25 (Mentored Quantitative Research Career DevelopmentAwards)
Available to individuals with quantitative (E.g., engineering, mathematics, computer science, etc.) backgrounds who wish to pursue biomedical research.
Training-Related Program Announcements
Small Grant Program for NIDDK K01/K08/K23 Recipients (R03)
In the final two years of the career development grant, K recipients may apply for small grant funding for additional development support for their research.
NIDDK Education Program Grants (R25)
The R25 program provides support for educational opportunities (E.g., workshops, classes) to engage students from undergraduate to graduate in research areas relevant to NIDDK.
K99/R00 NIH Pathways to Independence
The NIH has another opportunity for career development. This is an ideal award for talented postdoctoral candidates on the fast-track to a productive research career. Eligible applicants must have five-years or fewer of postdoctoral research experience and may not already have an independent faculty position. The first two years of the award, the K99 phase, are intended to be the mentored career-development phase. At the end of the second year, the applicant must have secured an independent tenure-track position to continue the final three years of the award as an R01. While this award does not require U.S. citizenship or permanent resident status, the applicant must be able to remain in the United States to conduct the full five years of the proposed work.
*All NIH fellowships and career development award mechanisms, except the K99/R00, require U.S. citizenship or permanent resident status.
Please visit our new KUH website
GOOGLE us at “NIH KUH home”
Contacts
Kidney, Urology & Hematology (KUH) Staff
Telephone: (301) 594-7717
Director, KUH
Robert A. Star, M.D.
starr@extra.niddk.nih.gov
Deputy Director, Clinical; Pediatric Neph Program & Renal Centers
Marva Moxey-Mims, M.D.
mimsm@extra.niddk.nih.gov
Deputy Director, Basic; Renal Physiology Program
Christian J. Ketchum, Ph.D.
ketchumc@extra.niddk.nih.gov
Director, Minority Health Program
Lawrence Y. Agodoa, M.D.
agodoal@extra.niddk.nih.gov
Epidemiology Program and U.S. Renal Data System
Paul W. Eggers, Ph.D.
eggersp@extra.niddk.nih.gov
Clinical PKD & Inflammatory Renal Diseases Programs
Michael F. Flessner, MD, PhD
flessnermf@niddk.nih.gov
Kidney Development & Repair & Regeneration Programs
Deborah Hoshizaki, Ph.D.
hoshizakid@niddk.nih.gov
Acute Injury and HIV Nephropathy Program
Paul L. Kimmel, M.D.
kimmelp@extra.niddk.nih.gov
Clinical Trials Program
John W. Kusek, Ph.D.
kusekj@extra.niddk.nih.gov
Urology and Kidney Cell Biology Programs
Christopher V. Mullins, Ph.D.
mullinsc@extra.niddk.nih.gov
National Kidney Disease Education Program (NKDEP)
Andrew S. Narva, M.D.
narvaa@extra.niddk.nih.gov
Kidney and Urology Training
Tracy L. Rankin, Ph.D.
rankint@mail.nih.gov
Genetics and Basic PKD Programs
Rebekah Rasooly, Ph.D.
rasoolyr@extra.niddk.nih.gov
Kidney Pathophysiology Program
Krystyna Rys-Sikora, Ph.D.
Krystyna.Rys-Sikora@nih.gov
NIH Center for Scientific Review (CSR)
The Review activities of the Center for Scientific Review (CSR) are organized into Integrated Review Groups (IRGs). Each IRG represents a cluster of study sections around a general scientific area. Here are 3 primary study sections for Kidney Research with the Digestive, Kidney and Urological Sciences (DKUS) Integrated Review Group (IRG):
- Cellular and Molecular Biology of the Kidney [CMBK] Reviews basic and applied aspects of normal and abnormal renal physiology, epithelial biology, cell biology, transport biology (including osmoregulation and osmosensing), hormone action and signal transduction, vascular biology, genetic disorders, cell-matrix interactions, biophysics, and bioenergetics.
- Pathobiology of Kidney Disease [PBKD] Reviews basic and clinical studies of kidney disease including pathophysiology, diagnosis, consequences and treatment of acute and chronic disorders of the kidney, and consequences of kidney disease and failure, as well as studies of the normal structure and function of the glomerulus.
- Urologic and Kidney Development & Genitourinary Diseases [UKGD] Reviews concerning the physiologic and pathophysiologic processes of the lower urinary tract, male reproductive organs, female pelvic floor, urolithiasis, and basic processes underlying upper and lower genitourinary organ development.
DKUS IRG CHIEF
Mushtaq Khan, D.V.M., Ph.D.
khanm@csr.nih.gov
Scientific Review Officers
CMBK
Bonnie Burgess-Beusse, Ph.D.
bb194m@nih.gov
PBKD
Atul Sahai, Ph.D
Atul.Sahai@nih.gov
UKGD
Ryan Morris, Ph.D.
morrisr@csr.nih.gov
Kidney Research National Dialogue
Kidney disease is a costly and major public health and medical problem. To best position our research community to identify the most robust paths forward, we are initiating an interactive web-based dialogue on kidney health and disease to elicit a wide range of ideas and opinions.
This dialogue will occur in three sequential phases over the next several months. FIRST, participants will identify critically important questions or objectives that could be addressed through basic and/or clinical research. The questions and objectives should be compelling, answerable, and have the greatest promise to advance scientific knowledge or improve public health. SECOND, participants will develop research strategies that address each of the research questions and objectives. All types of basic, translational, and clinical research should be considered. In some cases, fundamental groundwork may be needed before a larger question or objective can be approached. Thus, research strategies might include catalytic steps that surmount current barriers to progress. THIRD, the online dialogue will be edited and incorporated into a blueprint for kidney research and posted for public comment.
For more details contact KRND@nih.gov or kidneydialogue@nih.gov or GOOGLE “KUH KRND”.
Clinical Translational Research
Clinical Trials and Epidemiological Studies
NIDDK supports a wide range of clinical trials and epidemiological studies on chronic kidney disease. While many of these programs are solicited by NIDDK through initiatives, investigators may also develop their own ideas.
Areas of General Interest
- Clinical trials to prevent or slow chronic renal disease
- Epidemiology, prevention, and treatment of acute kidney injury
- Epidemiologic and genetic studies of ESRD patients
- Clinical trials to reduce mortality and morbidity in ESRD patients
- Epidemiology of chronic renal insufficiency, including CV disease
Mechanisms of Support
NIDDK will support investigator-initiated, multi-center studies exclusively through a two-step application process, with an implementation planning grant (U34) application followed by a clinical study cooperative agreement (U01) application.
If you have patients with Vesicoureteral Reflux (VUR)
RIVUR (Randomized Intervention for Vesicoureteral Reflux)
Multi-center trial of Trimethoprim-Sulfamethoxazole prophylaxis compared to placebo in children with VUR.
Study population:
Individuals between 2 months and 6 years of age with VUR and
- Diagnosed urinary tract infection (UTI) with either fever or associated symptoms
- Diagnosed with first UTI within 112 days prior to beginning study medication
- Treated for the first UTI for 7 or more days with an effective drug
For more information:
See www.ClinicalTrials.gov identifier NCT00405704
See http://www.cscc.unc.edu/rivur/
RIVUR Participating Sites:
Oregon Health and Science University, University of Oklahoma Health Sciences Center, Texas Children’s Hospital, Children’s Mercy Hospital, University of Wisconsin Children’s Hospital, Children’s Memorial Hospital, Cincinnati Children’s Hospital, University of Alabama, Children’s Hospital of Michigan, Akron Children’s Hospital, Children’s Hospital of Pittsburgh, Wake Forest University Baptist Medical Center, Women and Children’s Hospital of Buffalo, Children’s National Medical Center, Johns Hopkins School of Medicine, Penn State Hershey Medical Center, Children’s Hospital of Philadelphia, Alfred
Ongoing Studies/Currently Recruiting
- Oral vs. IV Iron Therapy in Chronic Kidney Disease
Single center study (Indiana University) investigating whether IV iron therapy in CKD is associated with more rapid decline in GFR than chronic oral iron therapy. - Randomized Intervention for Vesicoureteral Reflux (RIVUR)
Multicenter trial of Trimethoprim-Sulfamethoxazole prophylaxis compared to placebo in children with VUR (13 primary sites and 1 DCC). Age range 2 months – 6 years. Outcome measures will include frequency of UTI, changes in scarring measured by DMSA scan and development of antimicrobial resistance.
(www.ClinicalTrials.gov identifier NCT00405704) - Prospective Study of Chronic Kidney Disease in Children (CKiD)
Cooperative agreement-supported clinical study; 2 Clinical Coordinating Centers and 1 DCC.
Ongoing Studies/NOT Currently Recruiting
- Assessing Long Term Outcomes of Living Donation (ALTOLD) Multi-center prospective cohort study will address whether kidney donation increases the risk of developing ESRD and/or increases the risk of developing CV disease.
Participating sites: University of Minnesota (Hennepin County), Ohio State University, Mayo Clinic, University of Iowa, Johns Hopkins University, UCSF, University of Maryland. - Clinical Trial of FSGS in Children and Young Adults
Cooperative agreement-supported clinical trial; 3 primary centers and 1 DCC - Clinical Trial of FSGS in Children and Young Adults
Cooperative agreement-supported clinical trial; 3 primary centers and 1 DCC - Consortium for Radiologic Imaging Studies in PKD II (CRISP II)
Cooperative agreement-supported clinical study; 4 centers and 1 DCC - Angiotensin II Blockade in Chronic Allograft Nephropathy (ABCAN)
Cooperative agreement-supported clinical trial; single center at University of Minnesota - Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT)
Cooperative agreement clinical trial; 20 centers and 1 DCC - Family Investigation of Nephropathy and Diabetes (FIND)
Cooperative agreement-supported clinical study; 8 centers and 1 DCC - Chronic Renal Insufficiency Cohort (CRIC) Study
Cooperative agreement-supported prospective epidemiological study: 7 centers and 1 DCC - Frequent Hemodialysis Network (FHN)
Cooperative agreement supported clinical study: 2 sites and 1 DCC - HALT-PKD
Cooperative agreement-supported clinical study; 6 sites and 1 DCC - The NOCTURNAL TRIAL
1 primary site and 1 DCC
Repository
Central NIDDK Repositories
Data, Biosamples, and DNA for your research
The NIDDK Central Repositories store samples and data from NIDDK-funded clinical studies, which are made available to the community at the end of the study or when an interim phase is completed.
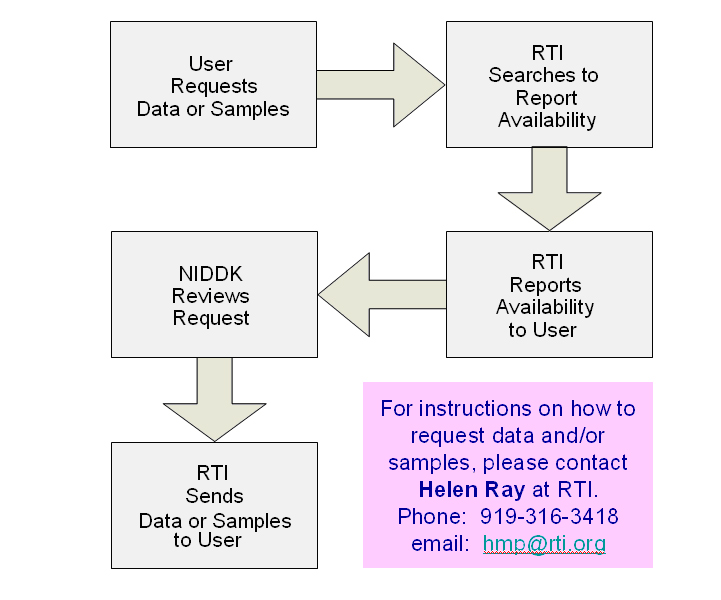
http://www.NIDDKrepository.org
Since 2005, the Repository has distributed data to 148 requesters!
Approved diabetes/kidney study requests:
| Study | Approved Requests |
|---|
| AASK | 7 |
| ATN | 2 |
| CRISP | 7 |
| DPP | 18 |
| DPT-1 | 6 |
| EDIC/DCCT | 25 |
| GoKind | 9 |
| HEMO | 9 |
| MDRD | 14 |