For Consumers
First Rapid Home-Use HIV Kit Approved for Self-Testing
 Get Consumer Updates by E-mail
Get Consumer Updates by E-mail
The OraQuick In-Home HIV Test will be available in stores and online (final packaging may be different). |
 Share this article (PDF 304 K)
Share this article (PDF 304 K)
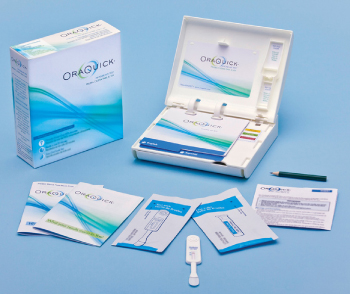
The Food and Drug Administration (FDA) has approved the OraQuick In-Home HIV Test, a rapid home-use HIV test kit that does not require sending a sample to a laboratory for analysis. The kit provides a test result in 20-40 minutes, and you can test yourself in your own home.
The kit, which tests a sample of fluid from your mouth, is approved for sale in stores and online to anyone age 17 and older.
FDA wants consumers to know that positive test results using the OraQuick test must be confirmed by follow-up laboratory-based testing. Also, the test can be falsely negative for reasons that include the occurrence of HIV infection within three months before testing. People who engage in behaviors that put them at increased risk of getting HIV—including having unprotected sex with new partners, or injecting illegal drugs—should be re-tested on a regular basis. They should not interpret a negative test to indicate that engaging in high risk behavior is safe.
According to the Centers for Disease Control and Prevention, about 1.2 million people in the U.S. are living with HIV infection, and about 1 in 5 of these people don't know that they are infected, increasing the chance that they will unknowingly spread the infection.
Elliot Cowan, Ph.D., in FDA's Division of Emerging and Transfusion Transmitted Diseases, describes the potential impact of this test and the messages that FDA wants to send consumers.
Q: Do you anticipate that the approval of OraQuick will impact HIV testing?
A: This test is targeted to people who would not otherwise be tested. There's a large group of people who are infected, and don't know it. And even if they are engaged in behaviors that would put them at risk of getting HIV, they may be reluctant to visit their doctor or a health care facility to be tested.
FDA predicts that the availability of the OraQuick In-Home HIV test will contribute measurably to public health by helping more infected individuals to become aware of their HIV status and thereby reducing HIV transmission.
Since 2002, FDA has approved a number of rapid HIV tests (tests that require no special equipment and provide results in as little as 20 minutes) that can be used by trained individuals outside of a traditional laboratory or clinic. The OraQuick In-Home HIV Test provides another testing option for people to learn their HIV status.
Q: How does the test work?
A: The test checks for antibodies to HIV. You swab your upper and lower gums for an oral fluid sample with the test device. That device is placed in a tube with a developing solution. After 20 to 40 minutes, one line will appear if the test is negative. Two lines indicate that HIV antibodies were detected and that you may be HIV positive. Follow-up confirmatory testing is then needed.
Q: So if it's positive, does that mean I definitely have HIV?
A: Not necessarily. What it does mean is that further testing is needed to confirm your HIV status. Look at this as a first step in HIV testing. No test is perfect—there will be false positives. Clinical studies for self-testing have shown that the OraQuick test will produce one false positive result out of about every 5,000 tests in uninfected individuals.
But if you get a positive result, it's very important that you see your healthcare professional or call the OraQuick Consumer Support Center, which has counselors available 24 hours a day to answer questions and provide local referrals for follow-up testing and care.
Q: And if it's negative, does that mean that I definitely don't have HIV?
A: No - and this is important for users of the test to understand. The test is not reliable at detecting HIV infection until at least three months after infection. In addition, even after three months, there can also be false negatives. Clinical studies by untrained consumers showed that the OraQuick test will produce about one false negative result out of every 12 tests performed in HIV infected individuals. It's also important never to use a negative test result to decide on whether to engage in behavior that puts you at risk for HIV infection.
Q: If there are antibodies in the mouth, does this mean I can pass HIV by kissing someone?
A: The antibodies are the body's reaction to the presence of the virus. They are not the virus itself. Body fluids that have been shown to contain high concentrations of the HIV virus are blood, semen, vaginal fluid, breast milk and other body fluids containing blood. So, only if there is blood in the saliva (for example, due to a cut in the mouth) could saliva possibly contain sufficient virus to cause an infection.
Q: What other tests are approved in the U.S. to test for HIV?
A: A complete list of the HIV test kits approved in the U.S. is available on the Vaccines, Blood and Biologics page at fda.gov.
This article appears on FDA's Consumer Updates page, which features the latest on all FDA-regulated products.