Medical Devices
FDA Public Health Notification: Updated Data on Mortality Associated with the Medtronic AneuRx® Stent Graft System
Issued: March 17, 2008
Dear Colleague:
This notification is to re-emphasize the need for continued surveillance of patients treated with endovascular grafts and to provide you with updated information on the mortality risks associated with the use of the AneuRx® Stent Graft System to prevent abdominal aortic aneurysm (AAA) rupture. This Public Health Notification focuses on the AneuRx® Stent Graft System as it is the only currently marketed device with a significant number of patients with long-term clinical follow-up at five years.
Recommendations
- Clinicians should consider the information available in the product labels and in the yearly clinical updates from Medtronic and other graft manufacturers when selecting treatment options for patients with AAA and in following previously treated patients. The clinical updates for three of the currently marketed AAA endovascular grafts can be found at: http://www.endologix.com/professionals/clinical/; http://www.goremedical.com/resources/dam/assets/AH1406EN6.EXC.MR.pdf; and http://www.medtronic.com/physician/aneurx/experience.html. The fourth clinical update can be obtained by request at http://www.cookmedical.com/home.do.
- Patients should be strongly encouraged to comply with the follow-up recommendations in the product labeling.
- Based on our findings, we recommend that the AneuRx® Stent Graft be used only in patients who can be treated in accordance with the instructions for use and who meet the appropriate risk-benefit profile. In determining the risk-benefit profile for patients with AAA disease and the appropriate treatment option, among the factors to consider are:
- Long-term AAA-related mortality, especially due to AAA rupture. The information below suggests that the average risk of late AAA-related mortality associated with AneuRx® exceeds that associated with open surgery.
- The experience of the institution or the physician. If open or endovascular surgery is performed in institutions or by physicians with little experience with open or endovascular AAA repair, the mortality rate may be considerably higher than average with that procedure.
- Surgical risk factors for the individual patient. Patients who have substantial surgical risk factors such as age and co-morbidities (e.g., cardiac, renal and pulmonary) may experience a higher than average mortality rate for open resection of AAA. For a 70-year old patient, for example, the rate could range from 2% with no risk factors to over 40% with multiple co-morbidities.
- The patient’s willingness to comply with the follow-up schedule for the endovascular graft.
Background Information
We have issued two notifications1-2 in the past identifying several serious adverse events, including aneurysm ruptures, in patients treated with the AneuRx® Stent Graft. Available data indicated that the risk of aneurysm-related overall mortality in patients with the AneuRx® graft was significantly greater than in those undergoing open surgical repair. Since then, we have worked with the manufacturer, Medtronic Vascular, to obtain more complete follow-up data for the premarket investigational cohort of patients who received the flexible model of the AneuRx® Stent Graft3.
New Information
Our earlier notifications suggested an increasing trend in aneurysm-related mortality among AneuRx® graft patients. We had estimated that late aneurysm-related mortality was approximately 0.4% per year. We now have additional, longer-term data that suggests that aneurysm-related mortality continues to increase after 3 years post-implant, reaching 1.3% by year 4 and 1.5% by year 5. These rates are substantially higher than the mortality rate for open surgical repair, which average 0.18% per year with a range of 0 % to 0.3 % per year.4-11 These mortality data for open surgical repair are comparable to that presented in the recently published results of the EVAR-1 trial.12
The following Kaplan-Meyer curve presents the results of this analysis:
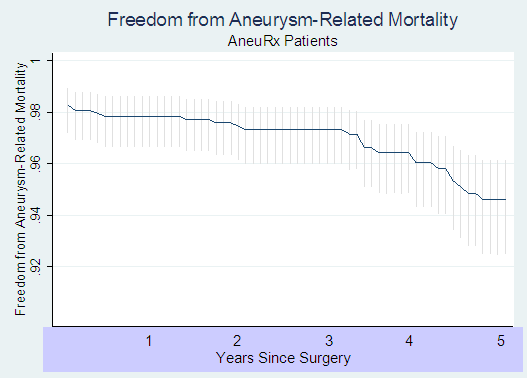
In addition, we now calculate, based on the latest information supplied by Medtronic, a mortality rate associated with the initial surgery of 2.3 % instead of the 1.5% originally calculated for the AneuRx ® patients.
These results differ somewhat from the information included in Medtronic’s clinical update because our analysis was based on a subset of patients. This subset consisted of the 931 patients (of a total study size of 1193) that were not designated “high risk” and who were implanted with the flexible design of the AneuRx ® stent graft, as this is more similar to the currently marketed device.
Reporting Adverse Events to FDA
FDA requires hospitals and other user facilities to report deaths and serious injuries associated with the use of medical devices. If you suspect that reportable adverse event was related to the use of an endovascular graft, you should follow the reporting procedures established by your facility.
We also encourage you to report adverse events related to endovascular grafts that do not meet the requirements for mandatory reporting. You can report directly to the FDA’s MedWatch Adverse Event Reporting program online, by phone at 1-800-FDA-1088, or by returning the postage-paid FDA form 3500 by mail to MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787 or fax at 1-800-FDA-0178.
Getting More Information
FDA Medical Device Public Health Notifications are available on the Internet. You can also be notified through email each time a new Public Health Notification is added to our web page. To subscribe, visit: http://service.govdelivery.com/service/subscribe.html?code=USFDA_39.
Sincerely yours,
Daniel G. Schultz, MD
Director
Center for Devices and Radiological Health
Food and Drug Administration
Appendix
Aneurysm-Related Mortality
As a follow-up to our previous public health notices, the risk of aneurysm-related mortality for the AneuRx ® endovascular graft was evaluated. Since aneurysm treatment is intended to mitigate the risk of aneurysm-related death, this outcome is significant in describing the effectiveness of endovascular repair of AAAs. Our assessment was focused on AneuRx ® based on the concerns described in the previous notices.
In addition to reviewing the information included in the AneuRx ® clinical update3, a subset analysis was conducted using the 931 study subjects (of a total study sample of 1193 subjects) who were not designated “high risk” and who were implanted with the flexible design of the AneuRx ® stent graft, as this device is more similar to the currently marketed device than the original stiff-body design used in the premarket study. These patients were considered good candidates for treatment with conventional open surgery. Certain categories of high risk patients (for surgical repair) were excluded from the study: American Society of Anesthesiology grade above IV; morbid obesity; acute renal failure or chronic dialysis; active systemic infection; less than one year of life expectancy; leaking aneurysm; aortic dissection; and aortic-iliac occlusive disease. The 931 study subjects were the same subset of patients used in the analysis reported in the 2003 public health notice.
A total of 295 deaths were identified in the study group as of April 27, 2007, 37 of which were judged to be AAA-related (12.5% of the total). We defined aneurysm-related death to include deaths from rupture of the AAA or from any procedure intended to treat the AAA. Additionally, if a death occurred within 1 month of any procedure intended to treat the AAA, it was presumed to be aneurysm-related, unless there was evidence to the contrary.
The following Kaplan-Meyer curve presents the results of this analysis:
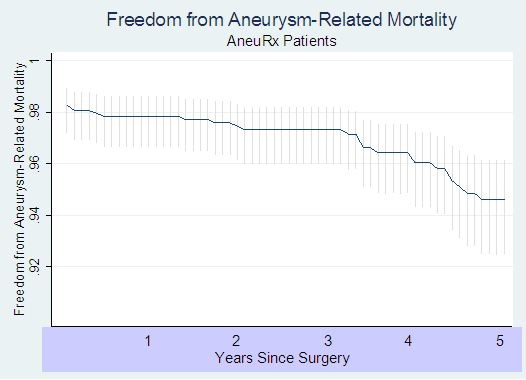
The initial (i.e. operative) aneurysm-related death rate was 2.26%. Following that, the rate rose from less than 0.4% per year of follow-up in each of the first 3 years to 1.3% per year in year 4 and 1.5% in year 5 (p=0.0003). A Kaplan-Meyer 5-year AAA-related death free survival rate was 94.7% (95% C.I. = 92.7% - 96.2%), which translates into a 5-year AAA-related death rate of 5.3% (95% C.I. = 3.8% - 7.3%).
Our estimates of 1.3% for AAA-related death rate in year 4 and 1.5% in year 5 is substantially higher than the rate of 0.2% per year for AAA-related deaths in subjects who receive open surgery, based on several individual studies, which suggest long-term mortality rates associated with open surgical repair of AAA ranging from 0 % to 0.3 % per year, and averaging 0.2% per year4-11. These mortality data for open surgical repair are comparable to that presented in the recently published results of the EVAR-1 trial.12
In addition, we now calculate, based on the latest information supplied by Medtronic, a mortality rate associated with the initial procedure of 2.3 % instead of the 1.5% originally calculated.
References
- FDA Public Health Notification: Problems with Endovascular Grafts for Treatment of Abdominal Aortic Aneurysm. Food and Drug Administration. April 27, 2001.
- FDA Public Health Notification: Updated Data on Mortality Associated with the Medtronic AVE AneuRx Stent Graft System. Food and Drug Administration. December 17, 2003.
- Medtronic Corporation. Investigational Plan: Medtronic AneuRx Endovascular Prosthesis Treatment of Abdominal Aortic Aneurysms
- Johnston KW, “Nonruptured Abdominal Aortic Aneurysm: Six-year Follow-up Results form the Multicenter Prospective Canadian Aneurysm Study”, J Vasc Surgery 20: 163-70, 1994.
- Lederle FA, et al, “Immediate repair compared with surveillance of small abdominal aneurysms”, N Eng J Med. 2002 May 9, 346(19):1437-44.
- Arko, et al, “Aneurysm-related death: Primary endpoint analysis for comparison of open and endovascular repair”, J Vasc Surg 36(2): 297-304, 2002.
- Hallet, et al, “Graft-Related Complications after Abdominal Aortic Aneurysm Repair: Reassurance from a 36-Year Population-based Experience”, J Vasc Surgery 25:277-286, 1997
- Biancari F, et al, “Durability of Open Repair of Infrarenal Abdominal Aortic Aneurysm: A 15-Year Follow-up Study”, J Vasc Surg 35: 87-93, 2002.
- Hertzer NR, et al, “Open Infrarenal Abdominal Aortic Aneurysm Repair: The Cleveland Clinic Experience from 1989 to 1998”, J Vasc Surg 35,: 1145-1154, 2002.
- Plate G, Hollier LA, O’Brien P, Pairolero PC, Cherry KJ, Kazmier FJ. Recurrent Aneurysms and Late Vascular Complications Following Repair of Abdominal Aortic Aneurysms. Arch Surg 120: 590-594, 1985.
- Williamson WK, Nicoloff AD, Taylor LM, Moneta GL, Landry GJ, Porter JM. Functional Outcome after Open Repair of Abdominal Aortic Aneurysm. J Vasc Surg 33: 913-920, 2001.
- Greenhalgh, RM, et al, Endovascular Aneurysm Repair versus Open Repair in Patients with Abdominal Aortic Aneurysm (EVAR trial 1): randomised controlled trial. The Lancet, June 2005; 1-8
If you have questions about this Notification, please contact FDA's Division of of Small Manufacturers, International and Consumer Assistance (DSMICA) by e-mail at dsmica@fda.hhs.gov or by phone at 1-800-638-2041 or 301-796-7100