General Information About Childhood Acute Lymphoblastic Leukemia (ALL)
Incidence and Epidemiology
Anatomy
Risk Factors for Developing ALL
Down syndrome
Inherited genetic polymorphisms
Overall Outcome for ALL
Current Clinical Trials
Fortunately, cancer in children and adolescents is rare, although the overall incidence of childhood cancer has been slowly increasing since 1975.[1] Children and adolescents with cancer should be referred to medical centers that have a multidisciplinary team of cancer specialists with experience treating the cancers that occur during childhood and adolescence. This multidisciplinary team approach incorporates the skills of the following health care professionals and others to ensure that children receive treatment, supportive care, and rehabilitation that will achieve optimal survival and quality of life:
- Primary care physician.
- Pediatric surgical subspecialists.
- Radiation oncologists.
- Pediatric medical oncologists/hematologists.
- Rehabilitation specialists.
- Pediatric nurse specialists.
- Social workers.
(Refer to the PDQ Supportive and Palliative Care summaries for specific information about supportive care for children and adolescents with cancer.)
Guidelines for pediatric cancer centers and their role in the treatment of pediatric patients with cancer have been outlined by the American Academy of Pediatrics.[2] Because treatment of children with acute lymphoblastic leukemia (ALL) entails many potential complications and requires intensive supportive care (e.g., transfusions; management of infectious complications; and emotional, financial, and developmental support), this treatment is best coordinated by pediatric oncologists and performed in cancer centers or hospitals with all of the necessary pediatric supportive care facilities. It is important that the clinical centers and the specialists directing the patient’s care maintain contact with the referring physician in the community. Strong lines of communication optimize any urgent or interim care required when the child is at home.
Dramatic improvements in survival have been achieved in children and adolescents with cancer.[1] Between 1975 and 2002, childhood cancer mortality has decreased by more than 50%. For ALL, the 5-year survival rate has increased over the same time from 60% to 89% for children younger than 15 years and from 28% to 50% for adolescents aged 15 to 19 years.[1,3] Childhood and adolescent cancer survivors require close follow-up because cancer therapy side effects may persist or develop months or years after treatment. (Refer to the PDQ summary on Late Effects of Treatment for Childhood Cancer for specific information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors.)
Incidence and EpidemiologyALL is the most common cancer diagnosed in children and represents 23% of cancer diagnoses among children younger than 15 years. ALL occurs at an annual rate of approximately 30 to 40 cases per million people in the United States.[4,5] There are approximately 2,900 children and adolescents younger than 20 years diagnosed with ALL each year in the United States.[5,6] Over the past 25 years, there has been a gradual increase in the incidence of ALL.[7]
A sharp peak in ALL incidence is observed among children aged 2 to 3 years (>80 cases per million per year), with rates decreasing to 20 cases per million for ages 8 to 10 years. The incidence of ALL among children aged 2 to 3 years is approximately fourfold greater than that for infants and is nearly tenfold greater than that for adolescents aged 16 to 21 years.
The incidence of ALL appears to be highest in Hispanic children (43 cases per million).[4,5] The incidence is substantially higher in white children than in black children, with a nearly threefold higher incidence of ALL from age 2 to 3 years in white children compared with black children.[4,5]
AnatomyChildhood ALL originates in the T- and B-lymphocytes in the bone marrow (see Figure 1).
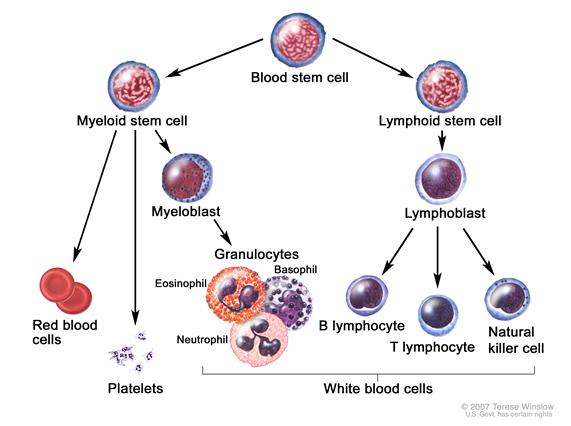
Marrow involvement of acute leukemia as seen by light microscopy is defined as follows:
- M1: Fewer than 5% blast cells.
- M2: 5% to 25% blast cells.
- M3: Greater than 25% blast cells.
Most patients with acute leukemia present with an M3 marrow.
Risk Factors for Developing ALLFew factors associated with an increased risk of ALL have been identified. The primary accepted risk factors for ALL include the following:
- Prenatal exposure to x-rays.
- Postnatal exposure to high doses of radiation (e.g., therapeutic radiation as previously used for conditions such as tinea capitis and thymus enlargement).
- Genetic conditions that include the following:
- Down syndrome.
- Neurofibromatosis.[8]
- Shwachman syndrome.[9,10]
- Bloom syndrome.[11]
- Ataxia telangiectasia.[12]
- Inherited genetic polymorphisms.
Children with Down syndrome have an increased risk of developing both ALL and acute myeloid leukemia (AML),[13,14] with a cumulative risk of developing leukemia of approximately 2.1% by age 5 years and 2.7% by age 30 years.[13,14]
Approximately one-half to two-thirds of cases of acute leukemia in children with Down syndrome are ALL. While the vast majority of cases of AML in children with Down syndrome occur before the age of 4 years (median age, 1 year),[15] ALL in children with Down syndrome has an age distribution similar to that of ALL in non–Down syndrome children, with a median age of 3 to 4 years.[16,17]
Patients with ALL and Down syndrome have a lower incidence of both favorable (t(12;21) and hyperdiploidy) and unfavorable (t(9;22) or t(4;11) and hypodiploidy) cytogenetic findings and a lower incidence of T-cell phenotype.[15-18] Approximately 50% of children with Down syndrome and ALL have a recurring interstitial deletion of the pseudoautosomal region of chromosomes X and Y that juxtaposes the first, noncoding exon of P2RY8 with the coding region of CRLF2. The resulting P2RY8-CRLF2 fusion gene is observed at a much lower frequency (<10%) in children with B-precursor ALL who do not have Down syndrome.[19,20]
Approximately 20% of ALL cases arising in children with Down syndrome have somatically acquired JAK2 mutations,[21-23] a finding that is uncommon among younger children with ALL but that is observed in a subset of primarily older children and adolescents with high-risk B-precursor ALL.[24] Almost all Down syndrome ALL cases with JAK2 mutations also have the pseudoautosomal region deletion and express the P2RY8-CRLF2 fusion gene.[19] Preliminary evidence suggests no correlation between JAK2 mutation status and 5-year event-free survival in children with Down syndrome and ALL.[22]
Inherited genetic polymorphismsGenome-wide association studies show that some germline (inherited) genetic polymorphisms are associated with the development of childhood ALL.[25] For example, the risk alleles of ARID5B are strongly associated with the development of hyperdiploid B-precursor ALL. ARID5B is a gene that encodes a transcriptional factor important in embryonic development, cell type–specific gene expression, and cell growth regulation.[26,27]
Some cases of ALL have a prenatal origin. Evidence in support of this comes from the observation that the immunoglobulin or T-cell receptor antigen rearrangements that are unique to each patient’s leukemia cells can be detected in blood samples obtained at birth.[28,29] Similarly, in ALL characterized by specific chromosomal abnormalities, data exist to support that patients had blood cells carrying the abnormalities at the time of birth with additional cooperative genetic changes acquired postnatally.[28-30]
In one study, 1% of neonatal blood spots (Guthrie cards) tested positive for the TEL-AML1 translocation, far exceeding the number of cases of TEL-AML ALL in children.[31] Other reports confirm [32] or do not confirm [33] this finding; nonetheless, this may support the hypothesis that additional genetic changes are needed for the development of this type of ALL. Genetic studies of identical twins with concordant leukemia further support the prenatal origin of some leukemias.[34]
Overall Outcome for ALLAmong children with ALL, more than 95% attain remission, and approximately 80% of patients aged 1 to 18 years with newly diagnosed ALL treated on current regimens are expected to be long-term event-free survivors.[35-40]
Despite the treatment advances noted in childhood ALL, numerous important biologic and therapeutic questions remain to be answered before the goal of curing every child with ALL with the least associated toxicity can be achieved. The systematic investigation of these issues requires large clinical trials, and the opportunity to participate in these trials is offered to most patients/families.
Clinical trials for children and adolescents with ALL are generally designed to compare therapy that is currently accepted as standard with investigational regimens that seek to improve cure rates and/or decrease toxicity. In certain trials in which the cure rate for the patient group is very high, therapy reduction questions may be asked. Much of the progress made in identifying curative therapies for childhood ALL and other childhood cancers has been achieved through investigator-driven discovery and tested in carefully randomized, controlled clinical trials. Information about ongoing clinical trials is available from the NCI Web site.
Current Clinical TrialsCheck for U.S. clinical trials from NCI's list of cancer clinical trials that are now accepting patients with childhood acute lymphoblastic leukemia. The list of clinical trials can be further narrowed by location, drug, intervention, and other criteria.
General information about clinical trials is also available from the NCI Web site.
References- Smith MA, Seibel NL, Altekruse SF, et al.: Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 28 (15): 2625-34, 2010. [PUBMED Abstract]
- Guidelines for the pediatric cancer center and role of such centers in diagnosis and treatment. American Academy of Pediatrics Section Statement Section on Hematology/Oncology. Pediatrics 99 (1): 139-41, 1997. [PUBMED Abstract]
- Hunger SP, Lu X, Devidas M, et al.: Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J Clin Oncol 30 (14): 1663-9, 2012. [PUBMED Abstract]
- Ries LA, Kosary CL, Hankey BF, et al., eds.: SEER Cancer Statistics Review, 1973-1996. Bethesda, Md: National Cancer Institute, 1999. Also available online. Last accessed October 05, 2012.
- Smith MA, Ries LA, Gurney JG, et al.: Leukemia. In: Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. Bethesda, Md: National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99-4649., pp 17-34. Also available online. Last accessed October 05, 2012.
- Dores GM, Devesa SS, Curtis RE, et al.: Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood 119 (1): 34-43, 2012. [PUBMED Abstract]
- Shah A, Coleman MP: Increasing incidence of childhood leukaemia: a controversy re-examined. Br J Cancer 97 (7): 1009-12, 2007. [PUBMED Abstract]
- Stiller CA, Chessells JM, Fitchett M: Neurofibromatosis and childhood leukaemia/lymphoma: a population-based UKCCSG study. Br J Cancer 70 (5): 969-72, 1994. [PUBMED Abstract]
- Strevens MJ, Lilleyman JS, Williams RB: Shwachman's syndrome and acute lymphoblastic leukaemia. Br Med J 2 (6129): 18, 1978. [PUBMED Abstract]
- Woods WG, Roloff JS, Lukens JN, et al.: The occurrence of leukemia in patients with the Shwachman syndrome. J Pediatr 99 (3): 425-8, 1981. [PUBMED Abstract]
- Passarge E: Bloom's syndrome: the German experience. Ann Genet 34 (3-4): 179-97, 1991. [PUBMED Abstract]
- Taylor AM, Metcalfe JA, Thick J, et al.: Leukemia and lymphoma in ataxia telangiectasia. Blood 87 (2): 423-38, 1996. [PUBMED Abstract]
- Hasle H: Pattern of malignant disorders in individuals with Down's syndrome. Lancet Oncol 2 (7): 429-36, 2001. [PUBMED Abstract]
- Whitlock JA: Down syndrome and acute lymphoblastic leukaemia. Br J Haematol 135 (5): 595-602, 2006. [PUBMED Abstract]
- Chessells JM, Harrison G, Richards SM, et al.: Down's syndrome and acute lymphoblastic leukaemia: clinical features and response to treatment. Arch Dis Child 85 (4): 321-5, 2001. [PUBMED Abstract]
- Zeller B, Gustafsson G, Forestier E, et al.: Acute leukaemia in children with Down syndrome: a population-based Nordic study. Br J Haematol 128 (6): 797-804, 2005. [PUBMED Abstract]
- Arico M, Ziino O, Valsecchi MG, et al.: Acute lymphoblastic leukemia and Down syndrome: presenting features and treatment outcome in the experience of the Italian Association of Pediatric Hematology and Oncology (AIEOP). Cancer 113 (3): 515-21, 2008. [PUBMED Abstract]
- Maloney KW, Carroll WL, Carroll AJ, et al.: Down syndrome childhood acute lymphoblastic leukemia has a unique spectrum of sentinel cytogenetic lesions that influences treatment outcome: a report from the Children's Oncology Group. Blood 116 (7): 1045-50, 2010. [PUBMED Abstract]
- Mullighan CG, Collins-Underwood JR, Phillips LA, et al.: Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat Genet 41 (11): 1243-6, 2009. [PUBMED Abstract]
- Harvey RC, Mullighan CG, Chen IM, et al.: Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood 115 (26): 5312-21, 2010. [PUBMED Abstract]
- Bercovich D, Ganmore I, Scott LM, et al.: Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down's syndrome. Lancet 372 (9648): 1484-92, 2008. [PUBMED Abstract]
- Gaikwad A, Rye CL, Devidas M, et al.: Prevalence and clinical correlates of JAK2 mutations in Down syndrome acute lymphoblastic leukaemia. Br J Haematol 144 (6): 930-2, 2009. [PUBMED Abstract]
- Kearney L, Gonzalez De Castro D, Yeung J, et al.: Specific JAK2 mutation (JAK2R683) and multiple gene deletions in Down syndrome acute lymphoblastic leukemia. Blood 113 (3): 646-8, 2009. [PUBMED Abstract]
- Mullighan CG, Zhang J, Harvey RC, et al.: JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A 106 (23): 9414-8, 2009. [PUBMED Abstract]
- de Jonge R, Tissing WJ, Hooijberg JH, et al.: Polymorphisms in folate-related genes and risk of pediatric acute lymphoblastic leukemia. Blood 113 (10): 2284-9, 2009. [PUBMED Abstract]
- Papaemmanuil E, Hosking FJ, Vijayakrishnan J, et al.: Loci on 7p12.2, 10q21.2 and 14q11.2 are associated with risk of childhood acute lymphoblastic leukemia. Nat Genet 41 (9): 1006-10, 2009. [PUBMED Abstract]
- Treviño LR, Yang W, French D, et al.: Germline genomic variants associated with childhood acute lymphoblastic leukemia. Nat Genet 41 (9): 1001-5, 2009. [PUBMED Abstract]
- Greaves MF, Wiemels J: Origins of chromosome translocations in childhood leukaemia. Nat Rev Cancer 3 (9): 639-49, 2003. [PUBMED Abstract]
- Taub JW, Konrad MA, Ge Y, et al.: High frequency of leukemic clones in newborn screening blood samples of children with B-precursor acute lymphoblastic leukemia. Blood 99 (8): 2992-6, 2002. [PUBMED Abstract]
- Bateman CM, Colman SM, Chaplin T, et al.: Acquisition of genome-wide copy number alterations in monozygotic twins with acute lymphoblastic leukemia. Blood 115 (17): 3553-8, 2010. [PUBMED Abstract]
- Mori H, Colman SM, Xiao Z, et al.: Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc Natl Acad Sci U S A 99 (12): 8242-7, 2002. [PUBMED Abstract]
- Zuna J, Madzo J, Krejci O, et al.: ETV6/RUNX1 (TEL/AML1) is a frequent prenatal first hit in childhood leukemia. Blood 117 (1): 368-9; author reply 370-1, 2011. [PUBMED Abstract]
- Lausten-Thomsen U, Madsen HO, Vestergaard TR, et al.: Prevalence of t(12;21)[ETV6-RUNX1]-positive cells in healthy neonates. Blood 117 (1): 186-9, 2011. [PUBMED Abstract]
- Greaves MF, Maia AT, Wiemels JL, et al.: Leukemia in twins: lessons in natural history. Blood 102 (7): 2321-33, 2003. [PUBMED Abstract]
- Möricke A, Reiter A, Zimmermann M, et al.: Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 111 (9): 4477-89, 2008. [PUBMED Abstract]
- Moghrabi A, Levy DE, Asselin B, et al.: Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood 109 (3): 896-904, 2007. [PUBMED Abstract]
- Pui CH, Campana D, Pei D, et al.: Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 360 (26): 2730-41, 2009. [PUBMED Abstract]
- Veerman AJ, Kamps WA, van den Berg H, et al.: Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997-2004). Lancet Oncol 10 (10): 957-66, 2009. [PUBMED Abstract]
- Salzer WL, Devidas M, Carroll WL, et al.: Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984-2001: a report from the children's oncology group. Leukemia 24 (2): 355-70, 2010. [PUBMED Abstract]
- Gaynon PS, Angiolillo AL, Carroll WL, et al.: Long-term results of the children's cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: a Children's Oncology Group Report. Leukemia 24 (2): 285-97, 2010. [PUBMED Abstract]


 Back to Top
Back to Top