Myth: Volcanoes and the Oceans are Causing Ozone Depletion
Source: World Meteorological Organization, Scientific Assessment of Ozone Depletion: 2002, WMO Global Ozone Research and Monitoring Project - Report No. 47, Geneva, 2002.
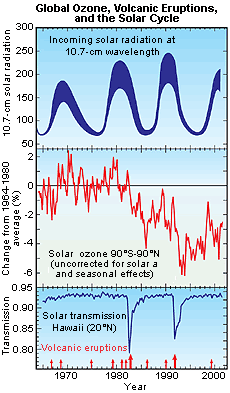
In the graphic depicted to the right, the top portion of the graph has a thick blue line that indicates changes in solar radiation (at the 10.7-cm wavelength). The highest incoming solar radiation between 1965 and 2000 occurred around 1990, when solar radiation at the 10.7-centimeter wavelength reached approximately 250 solar flux units, and the lowest radiation trends (at the 10.7-cm wavelength) occurred between 1975 and 1980, 1985 and 1990, and 1995 and 2000. The middle portion of the graph has a red line depicting change in total ozone between the latitudes 90 degrees South and 90 degrees North. Note that this graph did not correct changes in total ozone for solar and seasonal effects. The largest average percent change increase in total ozone between the 90 degree North and 90 degree South latitudes occurred in 1970 (approximately a 2% increase), while the largest negative average percent change (approximately 6%) occurred in 1994. The lower portion of the graph shows a thin blue line indicating solar transmission in Hawaii at 20 degrees North, and has red arrows showing volcanic eruptions that occurred between 1965 and 2002. Solar transmission greatly decreased between 1980 and 1985 and again between 1990 and 1995, when two large volcanic eruptions occurred.
Volcanic eruptions are powerful events, and they are capable of injecting hydrogen chloride (HCl) high into the atmosphere. Similarly, oceans produce large volumes of sea salt, which contains chlorine, on a daily basis. If these compounds accumulated in large quantities in the stratosphere, they might produce ozone depletion. However, for several reasons, we know that CFCs and other substances used in human activities are the primary sources of chlorine in the stratosphere.
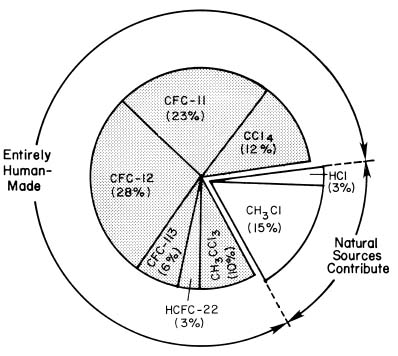
First, the vast majority of volcanic eruptions are too weak to reach the stratosphere, around 10 km above the surface. Thus, any HCl emitted in the eruption begins in the troposphere. Sea salt from the oceans is also released very low in the atmosphere. These compounds would have to remain airborne for 2-5 years to be carried to the stratosphere. However, both sea salt and HCl are extremely soluble in water, as opposed to CFCs which do not dissolve in water. Rain effectively scrubs the troposphere, removing both of these forms of chlorine. Steam in volcanic plumes can act the same way, removing HCl long before it reaches the ozone layer. Measurements have shown that concentrations of these substances vanish very rapidly as altitude increases. Neither sea salt from the oceans nor tropospheric-level volcanic eruptions (like Mt. Erebus in Antarctica) contribute significantly to stratospheric chlorine levels. Some sea life does produce methyl chloride, a more stable form of chlorine than sea salt, but its contribution is small, as explained below. The following graphic shows that natural sources only contribute 15% of methyl chloride to stratospheric chlorine levels, and natural sources of HCl contribute only 3%. The remaining sources of stratospheric chlorine are entirely human-made .
Source: World Meteorological Organization, Scientific Assessment of Ozone Depletion: 1998, WMO Global Ozone Research and Monitoring Project - Report No. 44, Geneva, 1998.
CFCs, on the other hand, do not dissolve in rain. In addition, no chemical processes have been found that aggressively remove them from the troposphere. In fact, one of the advantages of the CFCs was their stability. However, it is this very stability that poses a threat to the ozone layer.
Second, there is no historical record that shows significant increases in stratospheric chlorine following even the most major volcanic eruptions. Although El Chichon, in 1982, did increase concentrations of HCl in the stratosphere by 10%, that extra chlorine disappeared in about a year. When Mt. Pinatubo erupted in 1991, measurements found no increase in stratospheric chlorine. The dramatic increase in chlorine concentrations simply cannot be explained by a concurrent increase in volcanic activity.
Imagine that your birthday arrives, but you have no money in your savings account. You receive several gifts, but you can't quite remember whether your professor gave you a check. A call to the bank reveals that you have a balance of $100. One friend confirms she gave you $20, your mother reminds you that she sent $50, and your brother chipped in $30. By knowing the total and the amounts sent by everyone else, you conclude that your professor couldn't have sent you any money. Scientists performed exactly the same calculation. They could account for all of the sources of chlorine in the stratosphere, and only 3% was from HCl, probably from volcanoes. Another 15% of the chlorine entering the stratosphere derived from methyl chloride. However, fully 82% of stratospheric chlorine came from ODS, with 51% being carried there by CFC-11 and CFC-12.
Finally, Mt. Pinatubo did have an indirect effect because it emitted a large amount of aerosols, but it was short-lived. (Note that volcanic aerosols are tiny particles, not related in any way to consumer products called aerosols that have not used CFCs since the 1970s.) The aerosols essentially improved the efficiency of chlorine from CFCs, but they have already disappeared from the stratosphere. Thus, this temporary effect is not the root cause of ozone depletion.
The relationship between volcanic eruptions and ozone depletion is one of the most misunderstood in the realm of ozone science. Scientists seriously considered the impacts these awesome natural events might have on the ozone layer. They concluded, however, that ODS like CFCs are the real source of stratospheric chlorine, and thus ozone depletion.
Another
explanation![]() (search for "volcano"), with scientific references, is a
part of Robert Parson's ozone
depletion FAQ.
(search for "volcano"), with scientific references, is a
part of Robert Parson's ozone
depletion FAQ.![]()
![[logo] US EPA](https://cybercemetery.unt.edu/archive/oilspill/20120926062505im_/http://www.epa.gov/epafiles/images/logo_epaseal.gif)