
 |
| October 28, 2010 |
|
In 2008, an estimated 211,209 ED visits were made by children aged 12 or younger for adverse reactions to drugs. A little more than three fourths (76.8 percent) were made by children aged 5 or younger, while almost one fourth (23.2 percent) were made by children aged 6 to 12. Males accounted for a little more than half (54.5 percent) of the ED visits for adverse reaction to drugs among children. White children accounted for almost two thirds (63.3 percent) of these visits, Hispanic children for nearly one quarter (23.3 percent), and black children for about one tenth (10.8 percent).
Overall, most ED visits for adverse reactions among children involved one drug (91.6 percent) with a small percentage involving multiple drugs (8.4 percent). By age group, there were differences in the number of drugs involved with these visits. In comparison with their older counterparts, visits made by children aged 5 or younger had a higher percentage of visits involving one drug (92.7 vs. 87.7 percent; Figure 1). Visits made by patients aged 6 to 12 had a higher percentage of ED visits that involved two drugs than their younger peers (8.8 vs. 5.4 percent).
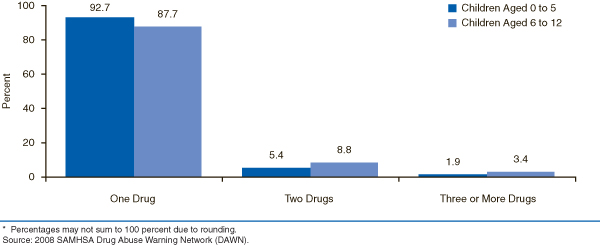
|
| Number of Drugs | Children Aged 0 to 5 | Children Aged 6 to 12 |
|---|---|---|
| One Drug | 92.7 | 87.7 |
| Two Drugs | 5.4 | 8.8 |
| Three or More Drugs | 1.9 | 3.4 |
| * Percentages may not sum to 100 percent due to rounding. Source: 2008 SAMHSA Drug Abuse Warning Network (DAWN). |
Anti-infection medications (e.g., penicillins, sulfonamides) accounted for the largest proportion of substances involved in ED visits for adverse reactions among children, accounting for a little more than half (55.4 percent) of visits among children aged 5 or younger and more than two fifths (43.3 percent) of visits among children aged 6 to 12 (Table 1). The most common anti-infection medications involved in these visits were penicillin-based drugs.
| Drug Category | Estimated Number of ED Visits, Children Aged 0 to 5 |
Percentage of ED Visits, Children Aged 0 to 5 |
Estimated Number of ED Visits, Children Aged 6 to 12 |
Percentage of ED Visits, Children Aged 6 to 12 |
|---|---|---|---|---|
| Total ED Visits | 162,261 | 100.0% | 48,948 | 100.0% |
| Anti-infection Medications | 89,968 | 55.4% | 21,190 | 43.3% |
| Cephalosporin Antibiotics | 13,296 | 8.2% | 2,868 | 5.9% |
| Macrolides | 9,723 | 6.0% | 2,694 | 5.5% |
| Penicillins | 55,894 | 34.4% | 9,861 | 20.1% |
| Sulfonamides | 5,132 | 3.2% | 3,194 | 6.5% |
| Immunologic Drugs | 33,541 | 20.7% | 4,176 | 8.5% |
| Nutritional Products | 12,927 | 8.0% | * | * |
| Central Nervous System Drugs (e.g., Pain Relievers, Drugs Used to Treat Anxiety and Insomnia) | 8,673 | 5.3% | 10,842 | 22.2% |
| Respiratory System Medications | 8,292 | 5.1% | 4,617 | 9.4% |
| Topical Agents (e.g., Germicides, Skin Cream) | 6,207 | 3.8% | 2,131 | 4.4% |
| Gastrointestinal System Medications | 1,814 | 1.1% | 1,136 | 2.3% |
| Hormones | 1,287 | 0.8% | 1,490 | 3.0% |
| Drugs for Metabolic Disorders | 748 | 0.5% | 703 | 1.4% |
| Psychotherapeutic Drugs (e.g., Antidepressants) | * | * | 3,006 | 6.1% |
| Cardiovascular System Medications | * | * | 474 | 1.0% |
| Drug Unknown | 1,945 | 1.2% | 1,111 | 2.3% |
| * Estimates are suppressed due to low statistical precision. Source: 2008 SAMHSA Drug Abuse Warning Network (DAWN). |
Excluding anti-infection medications, the remainder of the drugs involved in ED visits for adverse drug reactions among children varied by age group. About one fifth (20.7 percent) of such visits among patients aged 5 or younger were for immunologic drugs (e.g., viral vaccines) compared with 8.5 percent of visits for patients aged 6 to 12. Almost 1 in 12 visits (8.0 percent) among patients aged 5 or younger involved nutritional products (e.g., oral nutritional supplements). In contrast, more than one fifth (22.2 percent) of visits made by patients aged 6 to 12 involved central nervous system (CNS) drugs (e.g., pain relievers and drugs used to treat anxiety and insomnia) compared with a small percentage (5.3 percent) of visits among patients aged 5 or younger.
Most (93.5 percent) of the children aged 12 or younger who were taken to the ED for adverse reactions were treated and released, 4.1 percent were admitted to the hospital, and 2.4 percent had some other disposition (Figure 2). Of the visits that resulted in admission to the hospital, a little more than one third (35.7 percent) involved anti-infection medications, 10.3 percent involved nutritional products, and 7.9 percent involved CNS drugs (Figure 3).

|
| Disposition | Visits |
|---|---|
| Treated and Released | 93.5% |
| Admitted to the Hospital | 4.1% |
| Other Disposition | 2.4% |
| Source: 2008 SAMHSA Drug Abuse Warning Network (DAWN). |

|
| Drugs | Visits |
|---|---|
| Anti-infection Medications | 35.7% |
| Nutritional Products | 10.3% |
| Central Nervous System Drugs | 7.9% |
| Source: 2008 SAMHSA Drug Abuse Warning Network (DAWN). |
Thousands of children have an adverse reaction to drugs each year and, as a result, visit the ED. In 2008, the estimated number of ED visits for children aged 12 or younger exceeded 200,000; moreover, 3 in 4 of these visits were for children aged 5 or younger. A variety of drugs, including pharmaceutical and nutritional products, caused these reactions.
These findings point toward the need for increased education efforts focusing on parents, other family members, and temporary caregivers who may be in charge of administering medication. Pediatricians and pharmacists can play an important role in this effort by ensuring that parents and caregivers understand what symptoms and side effects to look for if they suspect an adverse reaction in their children. Moreover, it is critical that medical records are updated with any adverse reaction to prescription or OTC medications and that temporary caregivers are made aware of these known adverse reactions.
Finally, this report found that more than 9 in 10 ED visits resulted in these pediatric patients being treated and released. Trips to the ED can be a costly burden on the health care system. As Federal agencies seek ways to increase access to appropriate and high-quality health care while reducing costs, one potential avenue might be to enhance drug safety. To that end, the public health community can do its part by teaching parents and caregivers where and how to report adverse drug reactions through education and awareness campaigns. Health care providers also can ensure that adverse reactions are reported through appropriate channels. If reporting is bolstered, data quality on adverse reactions will be improved, leading to better health outcomes for children, families, and communities.
| The Drug Abuse Warning Network (DAWN) is a public health surveillance system that monitors drug related morbidity and mortality. DAWN uses a probability sample of hospitals to produce estimates of drug related emergency department (ED) visits for the United States and selected metropolitan areas annually. DAWN also produces annual profiles of drug related deaths reviewed by medical examiners or coroners in selected metropolitan areas and States. Any ED visit related to recent drug use is included in DAWN. All types of drugs—licit and illicit—are covered. Alcohol is included for adults when it occurs with another drug. Alcohol is always reported for minors even if no other drug is present. DAWN's method of classifying drugs was derived from the Multum Lexicon, Copyright 2008, Multum Information Services, Inc. The Multum Licensing Agreement can be found in DAWN annual publications. DAWN is one of three major surveys conducted by the Substance Abuse and Mental Health Services Administration's Center for Behavioral Health Statistics and Quality (SAMHSA/CBHSQ). For more information on other surveys, go to http://samhsa.gov/data/. SAMHSA has contracts with Westat (Rockville, MD) and RTI International (Research Triangle Park, NC) to operate the DAWN system and produce publications. For publications and additional information about DAWN, go to http://DAWNinfo.samhsa.gov/. |
| The DAWN Report is published periodically by the Center for Behavioral Health Statistics and Quality (formerly the Office of Applied Studies), Substance Abuse and Mental Health Services Administration (SAMHSA). All material appearing in this report is in the public domain and may be reproduced or copied without permission from SAMHSA. Additional copies of this report or other reports from the Center for Behavioral Health Statistics and Quality are available online: http://samhsa.gov/data/. Citation of the source is appreciated. For questions about this report, please e-mail: shortreports@samhsa.hhs.gov.
DAWN_023 |
This page was last updated on July 13, 2010. |