Medical Devices
Reduce - and Report - Enteral Feeding Tube Misconnections
By Courtney Jennings Millin, PhD, and Mary Brooks, MS, BSN, RN.
(Article reprinted from Nursing2010, November issue, p.60.)
ENTERAL FEEDING TUBES that are mistakenly connected to other applications may result in patient death or injury, as is widely known within the healthcare community. But in the last 4 years, only a few of these adverse events were reported to the FDA.¹²
Misconnections of enteral devices may be underreported because connecting feeding tubes to the wrong tubing, such as an I.V. or dialysis catheter, is often attributed to human error rather than device failure. This potential underreporting causes concern because the risk of enteral feeding tube misconnections could be further reduced by incorporating human factors assessment into device design. See Looking into human factors.
For these reasons, all adverse events and “near misses” should be reported to the FDA, whether they’re considered device or human error.
What can go wrong?
These adverse events reported to the FDA are examples of how multiple factors contribute to errors involving enteral feeding tube connections:
Case 1 - A patient with a newly inserted percutaneous endoscopic gastrostomy (PEG) tube also had a pre-existing peritoneal dialysis catheter, which a nurse accidentally accessed when providing enteral feeding. The nurse didn’t notice that the patient had more than one abdominal catheter.
Case 2 - A nurse connected enteral feeding formula to a gastrostomy tube port instead of the jejunostomy tube port as prescribed.
Case 3 - A patient requiring mechanical ventilation also had a nasogastric (NG) tube in place. After being transported to another department for a procedure, high-flow oxygen was accidentally connected to the NG tube. The patient subsequently underwent emergency surgery to repair the gastric perforation and colonic serosal tear resulting from the improper connection.
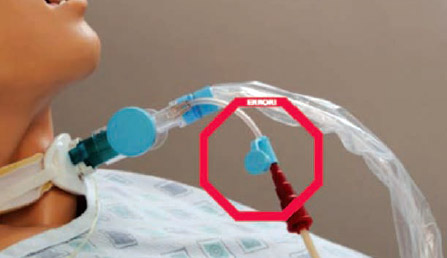
Case 4 - A pediatric night-shift nurse connected enteral feeding tubing to the lavage/irrigation port of a closed suction system. The nurse noted that the room was dark and that both connectors looked the same.
Case 5 - A patient’s enteral feeding formula was connected to an abdominal drainage tube that was mistaken for a PEG tube. The patient subsequently died.
These reports describe events that occurred due to both device and human error. Specific factors that contributed to these adverse events include:
- confusion about a new patient’s medical history
- confusion during the transfer of a patient to another department
- treatment situations that require nurses to perform multiple tasks simultaneously and quickly
- devices with different functions that appear similar
These contributing factors are not only device design problems but also systems problems. Evidence suggests that systems problems may be improved with human factors design changes.²,³
What’s being done?
Some manufacturers have been proactive in reducing the risk of enteral feeding tube misconnection by modifying their devices. These device modifications include the use of bright orange or purple tubing (solid and striped), “enteral-only” labels, and manufacturer-specific enteral-only or non-I.V. compatible connectors. Despite these device modifications, postmarket reporting of adverse events suggests that some of the current enteral-only or non-I.V. compatible connectors for feeding tubes don’t fully mitigate the risk of misconnection. These adverse event reports highlight some potential issues with enteral-only or non-I.V. compatible connectors.
Case 6 - Nurses reported a problem with the adaptor connector for an NG feeding tube set. They said that the adaptor could still be physically connected to inappropriate applications, resulting in leakage of fluids as well as potentially dangerous misconnections.
Case 7 - Staff at a facility were considering switching to an enteral-only feeding tube set. They evaluated one such product on the market and found that, althought the label stated that it was a non-I.V. compatible connection set, I.V. tubing was easily connected to the system.
These adverse events highlight the need for consensus on the design of small-bore connectors. Currently a joint working group of the International Organization for Standardization (ISO) and International Electrotechnical Commission (IEC), comprising manufacturers, clinical organizations, academic professionals, and government regulators, has begun to address this important issue.
The focus of the working group is to design a new small-bore connector that would prevent users from accidentally connecting one device to another system that could result in patient harm. This working group is establishing specific connector manufacturing design specifications and performance testing requirements for each medical device application.
What precautions can you take?
The risk of enteral feeding tube misconnection can be addressed with device design changes as well as education, awareness, and human factors assessment.4
Although the ISO and IEC working group has begun to address the problem at a device design level, clinicians and caregivers must be fully aware of steps they can take to reduce the risk of enteral feeding tube misconnections at the patient’s level. The following suggestions may help prevent errors.
- When connecting Luer-fitted devices, be vigilant to ensure proper connections and confirm that each device is clearly labeled.
- Don’t rely on color coding to prevent misconnection because colors aren’t always consistent or specific to device groups.
- Educate clinicians and patients in home-based or ambulatory care when new devices are introduced to the healthcare facility or home health agencies.
- Don’t modify feeding tube devices.
- Don’t force connections if device parts don’t seem to fit properly. Ill-fitting components should be a red flag that something isn’t right.
- Ensure good communication during patient transfer.
- Share with your colleagues the FDA device safety calendar, which displays clear examples of device misconnections, case studies, and Joint Commission safety tips.5 See Guard against this dangerous misconnection, which shows an example from the calendar of an enteral feeding tube erroneously connected to the lavage/irrigation port of a closed suction system.
- Follow your facility protocol for reporting adverse events and near misses. Also report any issues to the FDA through its MedWatch reporting system.
REFERENCES
- Eakle M, Gallauresi BA, Morrison A, Luer-lock misconnects can be deadly. Nursing. 2005;35(9):73.
- Simmons D, Graves K, Flynn EA. Threading needles in the dark: the effect of the physical work environment on nursing practice. Crit Care Nurs. 2009;32(2):71-74.
- Simmons D, Graves K. Tubing misconnections - a systems failure with human factors: lessons for nursing practice. Urol Nurs. 2008;28(6):460-464.
- Guenter P, Hicks RW, Simmons D. Enteral feeding misconnections: an update. Nutr Clin Pract. 2009;24(3):325-334.
- FDA. Medical Device Safety Calendar, 2009. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm134874.htm.
Although you need to support your healthcare facility’s adverse event-reporting policy, you may voluntarily report a medical device that doesn’t perform as intended by contacting MedWatch at 1-800-FDA-1088 (fax 1-800-FDA-0178) or online at http://www.fda.gov/Safety/MedWatch/HowToReport. Diane Dwyer, BSN, RN, who coordinates Device Safety, is a nurse consultant at the Center for Devices and Radiological Health at the FDA in Silver Spring, MD.
Courtney Jennings Millin is a staff fellow and biologist at the Center for Devices and Radiological Health at the FDA in Silver Spring, MD, where LCDR Mary Brooks is a senior regulatory review officer and nurse consultant. LDCR Brooks is an active duty officer serving in the U.S. Public Health Service.
Guard Against this Dangerous Misconnection
Looking into Human Factors
The FDA provides the following definition:
“Human factors (HF) is the study of how people use technology. It involves the interaction of human abilities, expectations, and limitations, with work environments and system design.
The term human factors engineering (HFE) refers to the application of human factors principles to the design of devices and systems. It’s often interchanged with the terms human engineering, usability engineering, or ergonomics.
The goal of HFE is to design devices that users accept willingly and operate safely in realistic conditions. In medical applications, HFE helps improve human performance and reduce the risks associated with use error.”