|
|
|||||||||||||||||||||||||||||||||||||||||||||
The Clinical Trial (CT) and Observational Study (OS) components of the WHI are interrelated and have been conducted at the same 40 clinical centers. The Clinical Trial (CT) and Observational Study (OS) scientifically sought answers to the long-waited questions on the benefits and risks of hormone therapy, changes in dietary patterns and calcium/vitamin D supplementation in disease prevention. The WHI CT/OS activities were performed at 40 clinical centers located throughout the United States. These clinical centers were funded through the contract mechanism. A single coordinating center, at the Fred Hutchinson Cancer Research Center, managed data collection and analysis. The CT/OS included a five-year recruitment period, which began in September 1993, and ended December 31, 1998, with a planned average of eight years of follow-up, and two years for data analysis.
Clinical Trial (CT)The randomized controlled clinical trial (CT) component of the WHI enrolled 68,131 postmenopausal women 50 to79 years of age. This trial had three interventions and women could enroll in one or more of the intervention components. The expected enrollment numbers for each component of the clinical trial was as follows:
The HORMONE THERAPY (HT) component examined the effect of hormonal therapy on prevention of coronary heart disease and osteoporotic fractures.
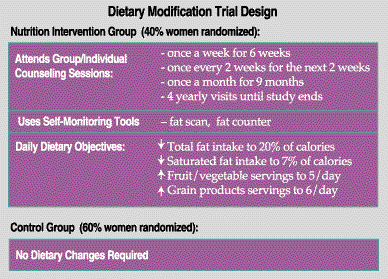
The DIETARY MODIFICATION (DM) component evaluated the effect of a low-fat dietary pattern on prevention of breast and colon cancer and coronary heart disease.
The CALCIUM/VITAMIN D SUPPLEMENTATION (CaD) component evaluated the effect of calcium and vitamin D supplementation on prevention of osteoporotic fractures and colon cancer. Women became eligible to join this component of the trial after being enrolled in either the Hormone Therapy (HT), Dietary Modification (DM) or both components for at 1 year.
Primary/Secondary Outcomes and Possible Adverse EffectsThe primary and secondary outcomes and possible adverse effects for the clinical trial at the time of the study design are listed in the table below: Clinical Trial Outcomes and Possible Adverse Effects
There were no known possible adverse effects for participation in the dietary modification (DM) components of the WHI clinical trial. Observational StudyWomen who were ineligible or unwilling to participate in the clinical trial were offered the opportunity to enroll in a concurrent long-term observational study (OS). This study sought to:
There were 93,676 women who joined this part of the study. Recruitment, follow-up and analysis for the OS was concurrent with the CT. |
|||||||||||||||||||||||||||||||||||||||||||||
 |
 |
 |
Please send us your feedback, comments, and
questions
by using the appropriate link on the page,
Contact the NHLBI.
Note to users of
screen readers and other assistive technologies: please report your problems
here.