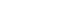About FDA
Medical Device Innovation Initiative White Paper
 CDRH Innovation Initiative
CDRH Innovation Initiative
February 2011
Center for Devices and Radiological Health
U.S. Food and Drug Administration
Table of Contents
Innovation and Medical Device Development
CDRH's Innovation Initiative
1. FACILITATE THE DEVELOPMENT AND REGULATORY EVALUATION OF INNOVATIVE MEDICAL DEVICES
1.1 Create a Priority Review Program for Pioneering Technologies (the Innovation Pathway)
1.2 Streamline the de novo Pathway
2. STRENGTHEN THE U.S. RESEARCH INFRASTRUCTURE AND PROMOTE HIGH-QUALITY REGULATORY SCIENCE
2.1 Establish a Voluntary Third-Party Certification Program for U.S. Medical Device Test Centers
2.2 Create a Publicly-Available Core Curriculum for Medical Device Development and Assessment
2.3 Leverage Device Experience and Data Collected Outside the United States
2.4 Develop New Science
3. PREPARE FOR AND RESPOND TO TRANSFORMATIVE INNOVATIVE TECHNOLOGIES AND SCIENTIFIC BREAKTHROUGHS
3.1 Horizon Scanning
3.2 CDRH Network of Experts
EXECUTIVE SUMMARY
The Food and Drug Administration's (FDA) Center for Devices and Radiological Health (CDRH or the Center) is responsible for advancing public health and facilitating innovation to help bring novel technologies to market and make the medical devices that are already on the market safer and more effective. As part of this Medical Device Innovation Initiative, CDRH is outlining additional actions the Center might take to encourage innovation, streamline regulatory and scientific device evaluation, and expedite the delivery of novel, important, safe and effective innovative medical devices to patients.
The Innovation Initiative proposes actions CDRH could take to help accelerate and reduce the cost of development and regulatory evaluation of innovative medical devices safely and based on sound science. These actions include:
- Facilitate the development and regulatory evaluation of innovative medical devices by:
- Establishing the Innovation Pathway - a priority review program for pioneering medical devices; and
- Streamlining the de novo pathway.
- Strengthen the U.S. research infrastructure and promote high-quality regulatory science by:
- Establishing a voluntary third-party certification program for U.S. medical device test centers;
- Creating a publicly-available core curriculum for medical device development and assessment;
- Leveraging device experience and data collected outside the United States; and
- Advancing regulatory science for medical devices through prioritizing scientific research, establishing public-private partnerships, collaborating with other government agencies, and holding public workshops.
- Prepare for and respond to transformative innovative technologies and scientific breakthroughs by:
- Enhancing CDRH's current horizon scanning process by adopting emerging horizon scanning methods, seeking public input to identify important and innovative medical device technologies as they arise, and periodically reporting its horizon scanning findings to the public; and
- Developing a Network (or Networks) of Experts to serve as a resource to assist in addressing scientific questions about emerging technologies with which our reviewers might not be immediately familiar.
CDRH is seeking public comment on all of the above actions and the proposals set out in the Medical Device Innovation Initiative through an open public docket and will be hosting a public meeting to solicit stakeholder feedback at our White Oak, Maryland, campus on March 15, 2011.
BACKGROUND
The United States is the global leader in medical device innovation and CDRH is committed to assuring that American patients have timely access to important new technologies and next-generation products without compromising their safety. Each year, millions of American patients benefit from innovative medical devices that reduce suffering, treat previously untreatable conditions, extend lives, and improve public health.
CDRH is responsible for advancing public health by facilitating innovation to help bring novel technologies to market and make the medical devices that are already on the market safer and more effective. Recently, the Center announced 25 actions it will take in 2011 to strengthen its most widely-used premarket review process - the 510(k) program - and reduce uncertainty in its use of emerging science to foster innovation and improve the predictability, consistency and transparency of its decision making.1 These actions will not only improve the safety and effectiveness of medical devices but also increase the ability of innovating companies to attract investors, estimate costs, and more quickly bring products to market.
As part of this Medical Device Innovation Initiative, CDRH is proposing additional actions the Center could take to encourage innovation, streamline regulatory and scientific device evaluation, and expedite the delivery of novel, important, safe and effective innovative medical devices to patients. Because improving the predictability of our premarket review programs is our number one priority, CDRH will proceed in a manner that does not delay the implementation of critical actions to reduce uncertainty and that does not adversely impact our premarket review performance. Instead, we will implement the Innovation Initiative to the extent practical given current resources, and would consider expanding the Initiative should additional resources become available.
In light of our commitment to meeting our Medical Device User Fee Act performance goals and improving the predictability of our current regulatory processes, expending significant resources to implement the Innovation Initiative is a luxury we cannot afford. Although, given current resources, we cannot yet make a radical overhaul of our review processes; we can lay the foundation for the new paradigm we are proposing herein.
CDRH is seeking public comment on the proposals contained in this report through an open public docket and will be hosting a public meeting to solicit stakeholder feedback at our White Oak, Maryland, campus on March 15, 2011.
Innovation and Medical Device Development
New scientific discoveries or novel ideas are often at the root of innovative medical device development - whether the product is a transformative technology, a modified version of an already marketed model, or a novel application of existing tools or scientific approaches. The regulatory process affects a significant portion of the device development pathway. To the extent it is feasible, regulatory pathways should accommodate and facilitate the iterative, cyclical nature of device design and development. They should also account for the inevitable cycles of design-prototype-test-redesign that are inherent to the development process.
CDRH has used a total product life cycle model (see Figure 1) to illustrate the iterative nature of medical device design and development and to highlight the importance of incorporating user needs and device experience into next-generation device development.
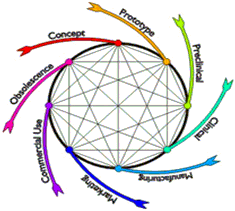
Figure 1. The Total Product Life Cycle approach to medical device development and regulation is shown. Medical device development is an iterative process that rapidly incorporates preclinical, clinical, and manufacturing experience into next-generation concept and design.
A large portion of a device's total product life cycle is occupied by product development from concept to marketing. The pathway to successful device development is cyclical and iterative as ideas are prototyped, tested, improved, re-tested, optimized and finalized. The device development pathway is a continuum with feedback loops and device modifications (Figure 2). Although portrayed as a compartmentalized process with distinct phases - such as pre-clinical and clinical - steps in device development overlap and portions may need to be repeated as testing and user experience are incorporated into product modifications and the device moves closer to its marketed form. And, product evaluations and modifications continue to occur even after a product reaches the market.
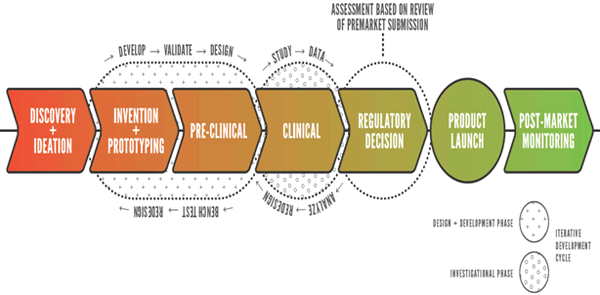
Figure 2. The medical device development pathway from discovery and ideation to product launch and post market monitoring is shown. The regulatory process affects a significant portion of the device development pathway and should accommodate the iterative, cyclical nature of device design and development.
CDRH's current regulatory pathways are designed to accommodate the incremental improvements manufacturers make to their marketed devices. The regulatory process allows manufacturers to modify existing devices and submit supporting data for regulatory review on a shortened timeframe. The 510(k) process facilitates access to modified versions of marketed lower-risk products by providing a streamlined review pathway for new devices proven to be "substantially equivalent" to legally marketed "predicate" devices. This pathway promotes evolutionary enhancements by providing a quicker pathway to market for newer versions of legally marketed lower-risk devices. Similarly, manufacturers may submit certain changes to devices approved under the premarket approval (PMA) process as PMA supplements that leverage previous device testing and experience where appropriate. The Center has also created certain resource-intensive review processes to reduce Agency decision times while still allowing for adequate assessment of an application. These programs include "real-time review", wherein CDRH will issue a decision on a PMA supplement generally within five business days of meeting with the sponsor, and "interactive review", which facilitates the efficient and timely review and exchange of regulatory and scientific information between CDRH and the sponsor.
CDRH recognizes that transformative innovative devices typically present new scientific and regulatory challenges. The Innovation Initiative supports the development of innovative products by addressing some of the barriers that can impede a product's timely progress to market.
The Innovation Initiative proposes actions CDRH could take to help accelerate the development and regulatory evaluation of innovative devices safely and based on sound science. These actions are:
- Facilitate the development and regulatory evaluation of innovative medical devices;
- Strengthen the U.S. research infrastructure and promote high-quality regulatory science; and
- Prepare for and respond to transformative innovative technologies and scientific breakthroughs.
1. FACILITATE THE DEVELOPMENT AND REGULATORY EVALUATION OF INNOVATIVE MEDICAL DEVICES
1.1 Create a Priority Review Program for Pioneering Technologies (the Innovation Pathway)
Recognizing the important benefits truly innovative medical devices have on the public health of Americans, CDRH proposes to establish a priority review program - the Innovation Pathway - for eligible innovative products.
CDRH has long recognized the importance of facilitating innovation and expediting the review of important new technologies. The expedited review process for medical devices was first established in 1994, and most recently described in a 2008 FDA Guidance entitled "Expedited Review of Premarket Submissions for Devices", which incorporated changes under the Food and Drug Administration Amendments Act of 2007 (FDAAA)(Public Law 110-85).2
A device is considered appropriate by FDA for expedited review if it:
- is intended to treat or diagnose a life-threatening or irreversibly debilitating disease or condition, and
- addresses an unmet medical need, as demonstrated by any oneof the following:
- The device represents a breakthrough technology3 that provides a clinically meaningful advantage over existing technology;
- No approved alternative treatment or means of diagnosis exists;
- The device offers significant, clinically meaningful advantages over existing approved alternative treatments; or
- The availability of the device is in the best interest of patients.
In the six-year period beginning with 2005 and ending with 2010, approximately 7% of PMA submissions, representing 23 out of 314 applications received, were granted expedited review status. Expedited review times are typically longer than standard review times and have not reliably met the targets FDA agreed to as part of Medical Device User Fee Act (MDUFA) negotiations, primarily due to the unique regulatory and scientific challenges presented by devices that are granted expedited review status. Nevertheless, compared to what would have occurred under the standard review program, expedited review has shortened the time to market for a number of important innovative technologies including drug-eluting coronary stents, implantable pacemakers, vision and hearing systems, and continuous glucose monitors.
The Innovation Pathway (Figure 3) is intended to provide earlier investment of Center time and resources in devices that are truly pioneering technologies and that have the potential to revolutionize patient care or health care delivery. We anticipate that the devices reviewed under this pathway may raise scientific and regulatory questions that are novel, challenging and resource-intensive. While it is critically important to take steps to facilitate the development of transformative innovative devices, we also recognize the importance of meeting our commitments under MDUFA. Therefore, the number of devices that we would be able to accommodate under the Innovation Pathway would depend on available resources. We would closely monitor our resources so that our performance and commitments for the review of other devices are not adversely affected, thus avoiding unintended consequences for devices reviewed under other pathways.
Figure 3. The Innovation Pathway recognizes the unique nature of transformative innovative product development. By front-loading critical aspects, such as identifying appropriate clinical endpoints and key scientific questions, and seeking advice from external experts, we can provide a more timely and efficient regulatory review process.
CDRH proposes that, to be eligible for consideration for the Innovation Pathway, the Center would have to determine that the device is radically different from any legally marketed medical device in the United States in its underlying technology or manner of use, and is designed to meet at least one of the following criteria:
- significantly improve upon currently available treatments or diagnostics for life-threatening or irreversibly debilitating diseases or conditions;
- treat or diagnose a life-threatening or irreversibly debilitating disease or condition for which no approved or cleared alternative treatment or means of diagnosis exists;
- address an unmet public health need as identified by the Council on Medical Device Innovation; or
- address an issue relevant to national security such as vaccine development and medical counter measures.
Although the Innovation Pathway is a proposal for which CDRH seeks public comment, the Center has accepted a pilot submission into the program: a revolutionary brain-controlled upper-extremity prosthetic designed to restore near-natural arm, hand and finger function to patients suffering from spinal cord injury, stroke or upper-extremity amputation. The arm system, funded by the Defense Advanced Research Projects Agency (DARPA), will use a microchip implanted on the surface of the brain to record neuronal activity and decode the signals to actuate motor neurons that control the prosthesis.
CDRH proposes that additional candidate devices for the Innovation Pathway may be identified in one of two ways: 1) at the request of the sponsor; or 2) at the suggestion of a CDRH employee or manager with the permission of the sponsor. No submissions would be considered for the Innovation Pathway without the explicit consent of the sponsor. The Center Science Council, which is described in greater detail below, would meet regularly to evaluate applications to the Innovation Pathway and would communicate a decision to the sponsor within 30 days of the application submission date. Decisions would be based on the revolutionary nature of the device, how well the submission meets the criteria listed above, and available and anticipated Center resources. Because of the innovative and transformative nature of the devices eligible for this pathway, it is expected that devices reviewed under this pathway generally will be PMA, PMA supplement, and de novo submissions.
The Innovation Pathway would have the following key features designed to meet the unique requirements of transformative medical device development and regulatory review:
- Oversight by the Center Science Council (CSC)4 - The CSC, a new oversight body currently being developed within CDRH, will be comprised of a cross-disciplinary group of CDRH senior managers and experienced review staff. The CSC would monitor the device development and review processes from the date of acceptance into the Innovation Pathway until the date of regulatory approval (or removal from the Innovation Pathway). As for other submissions, a primary review team would be assigned; however, under the Innovation Pathway, the primary review team would be assigned earlier in the development process and the team and management would regularly update the CSC on the progress of the submission, unresolved regulatory or scientific challenges, or proposed changes to prior policies or decisions. Early CSC involvement should lead to quicker resolution of difficult scientific issues, early recognition of the need for additional expertise outside the Center, and a reduction in unnecessary delays. In addition, the Center created a new position - Associate Director for Technology and Innovation - to work with the CSC and oversee the Center's innovation activities;
- Early Identification of Needed Expertise - Early in the Innovation Pathway process, the specific subject matter experts needed for scientific and regulatory evaluation of the submission would be identified. When the required expertise does not exist in CDRH, CDRH would seek to locate the appropriate expertise outside the Center, including from CDRH's Network of Experts (see below);
- Assignment of a "Case Manager" - Each product accepted into the Innovation Pathway would be assigned a case manager. Case managers would help sponsors navigate the Innovation Pathway process by coordinating Center actions for review of their device submissions, ensuring timely information exchange, and reporting directly to the CSC;
- Development of an Innovation Pathway Memorandum - The memorandum, developed through an interactive assessment process with the sponsor, would describe a proposed roadmap and timeline for device development, clinical assessment, and regulatory review. Delays and uncertainty would be minimized by identifying and addressing difficult, unresolved regulatory science questions (such as appropriate clinical trial endpoints) during the early Innovation Pathway stages. The Innovation Pathway Memorandum would generally be completed within 120 days from acceptance into the Innovation Pathway;
- Frequent Communication with the Sponsor - The sponsor and review team would communicate regularly throughout the development process to address questions or issues that arise, develop the clinical trial protocol, engage during premarket review or discuss other scientific or regulatory challenges along the way. The sponsor and review team would also meet periodically in person or via teleconference as needed
- Creation of Flexible Clinical Trial Protocols - Clinical trial protocols developed through an interactive assessment process would anticipate the need for iterative device testing and redesign, as appropriate, and may employ tools to best leverage available data and minimize delays. For example, multiple stages of clinical evaluation (such as feasibility and pivotal trials) may potentially be performed under a single protocol that allows for a phased-in approach. Iterative clinical trial designs may be employed when treatment effects are uncertain given the novelty of the technology; and
- Established Timeframes for Regulatory Review - Once the device completes the preclinical and clinical stages of development, it would be submitted for regulatory review. Given significant senior management and review team involvement throughout the device development process, CDRH proposes that reviewers would have 150 days to complete their review - which is approximately half the time they take to review most PMAs.
The proposed Innovation Pathway is designed to facilitate the scientific and regulatory evaluation of transformative innovative products and invest Center time and resources in these products earlier in the review process. Enrollment in the Innovation Pathway would not change the scientific or regulatory standards that CDRH would use to evaluate device submissions and determine their appropriateness for marketing. Instead, the Innovation Pathway would recognize the challenges of developing transformative innovative devices and increases the commitment of Center resources to their development and evaluation.
1.2 Streamline the de novo Pathway
The de novo classification process was created5 to provide a mechanism for the classification of certain lower-risk devices for which there is no predicate. The de novo classification process is intended to apply to lower-risk devices that are classified into class III through the 510(k) process. The de novo process is most applicable when the risks of a device are well-understood and appropriate special controls can be established to mitigate those risks.
As outlined in the 510(k) Working Group Report,6 current implementation of the de novo pathway is inefficient, unpredictable, and underutilized. From 2005 to 2009, CDRH reviewed 59 de novo submissions out of the more than 20,000 510(k) submissions received. In January 2011, CDRH recommended steps it will take to streamline implementation of the de novo process including issuing draft guidance by September 30, 2011.7
CDRH intends for this guidance to:
- Streamline 510(k) submissions and de novo petitions for eligible devices;
- Clarify the criteria for de novo eligibility; and
- Provide a more efficient process for de novo review.
Importantly, the development of de novo guidance and streamlining the de novo process is intended to increase the efficiency and predictability of regulatory review for low- and moderate-risk devices that lack an appropriate legally marketed predicate.
2. STRENGTHEN THE U.S. RESEARCH INFRASTRUCTURE AND PROMOTE HIGH-QUALITY REGULATORY SCIENCE
Innovative medical device development is facilitated by a solid research infrastructure that provides the scientific community with the tools and mechanisms to perform foundational research, develop new research methodologies, and enhance research collaboration and communication between different disciplines. Strengthening regulatory science - the science of developing new tools, standards and approaches to assess the safety, efficacy, quality and performance of FDA-regulated products - would serve to foster innovation, reduce the time and cost to meet regulatory evidentiary requirements, and improve the efficiency and quality of device manufacturing. In addition, having institutions and investigators skilled in good clinical practices can help assure proper clinical trial conduct, thereby also assuring patient protection and data integrity. A weak research infrastructure and underdeveloped regulatory science impedes the development of innovative devices because the tools necessary to efficiently evaluate their potential may not exist or the data collected from a clinical trial may be of insufficient quality.
The actions CDRH proposes to take to strengthen the U.S. device research infrastructure and promote regulatory science are as follows:
2.1 Establish a Voluntary Third-Party Certification Program for U.S. Medical Device Test Centers
Certifying medical device clinical trial centers would strengthen the U.S. research infrastructure by helping sponsors to more easily identify high quality test centers for medical device development and assessment while providing greater assurance of patient safety. Certified test centers would bring together the key scientific expertise required to more efficiently and safely develop and test innovative medical devices consistent with the iterative nature of device design, testing, and redesign. Sponsors, clinical investigators and Institutional Review Boards (IRBs) must continue to comply with all applicable FDA regulatory requirements.8 CDRH expects these test centers would be able to identify and correct device shortcomings quickly, thereby minimizing patient exposure to significant and unnecessary safety risks. In addition, because of their expertise and established safety records, CDRH would consider permitting these certified test centers to conduct first-in-human studies at earlier stages in the development process. In some cases, test centers may be able to acquire a "Center of Excellence" distinction related to expertise in specific areas (such as diabetes, wireless technologies, etc.). CDRH would consider using a third-party certification approach and implement such a program to the extent resources permit.
Test centers that believe they meet the certification criteria may volunteer for third-party certification at their own discretion. A clinical trial test center would be considered worthy of third-party certification if it met certain criteria, such as the following (some criteria overlap with mandatory FDA regulatory requirements):
- The center has access to necessary device development expertise through a formal relationship with a medical device design and engineering "academic" (i.e. non-industry) center;
- The center has a robust clinical program and diverse expertise to anticipate and manage medical issues that may arise during the course of clinical studies, particularly for those sites participating in early clinical trials and/or studying implanted devices;
- The center has a robust safety monitoring system, such as timely and effective reporting of adverse events, for significant risk studies; and
- The center demonstrates expertise in clinical trial design and conduct as well as human subject protection and data integrity training, expertise, and oversight consistent with Good Clinical Practices.
CDRH recognizes that currently there may be a limited number of institutions that are able to meet all of the criteria listed above, and that there may also be value to providing certification for institutions that conduct high quality clinical studies that assure data integrity and human subject protection. Therefore, CDRH would consider creating a two-tiered certification program. The first tier would include test centers that meet all the above-listed criteria (i.e., centers with both in-house device development and clinical assessment expertise); the second tier would include test centers that meet criteria 2 - 4 (i.e., centers with in-house clinical assessment expertise only). This two-tiered approach would be more resource-intensive, as more test centers would meet the eligibility criteria. CDRH is seeking public comment on the two-tiered versus single-tiered approach as well as establishing a voluntary certification program generally.
2.2 Create a Publicly-Available Core Curriculum for Medical Device Development and Assessment
Currently there are few institutions that possess all the necessary expertise to design, test and clinically evaluate devices, identify the root causes of adverse events and device malfunctions, develop iterative device designs, and navigate the regulatory process; even fewer offer curricula in these disciplines. To facilitate the widespread availability of educational programs in device development and assessment to train the next generation of innovators and help keep the U.S. the leader in medical device innovation, CDRH would work with academia, industry, and the health care community to develop a publicly-available core curriculum covering the areas of device design and engineering, pre-clinical testing, clinical evaluation, regulatory processes and post-market monitoring.
2.3 Leverage Device Experience and Data Collected Outside the United States
CDRH recognizes that a significant portion of medical device research occurs outside the United States, and CDRH accepts foreign clinical data that complies with FDA regulatory criteria.9
Historically, the applicability of data developed outside the United States has been limited due to key deficiencies, namely, insufficient study quality and/or data integrity, or lack of sufficient demographic and clinical information to determine applicability to the U.S. population. As part of its Innovation Initiative, CDRH would develop a guidance document describing the Center's recommendations for the criteria and circumstances under which foreign test centers should develop data to be used in support of U.S. device marketing applications. In addition, CDRH would explore the possibility of extending its voluntary third-party certification program for clinical testing sites outside the United States, resources permitting, from which CDRH would routinely accept clinical data.
CDRH has been and continues to be actively engaged in regulatory science research. In particular, scientists in CDRH's Office of Science and Engineering Laboratories (OSEL) pursue a broad portfolio of research and training activities, including:
- Product testing;
- Development of test methods for CDRH, industry, and academic use;
- Scientific investigations on emerging technologies;
- Participation in national and international standards development;
- Scientific and technical training of CDRH staff members; and
- Maintenance of laboratory collaborations and relationships with scientific researchers in academia and other Federal laboratories.
OSEL is also working on a number of projects to strengthen regulatory science, including:
- Novel imaging techniques to produce real-time, high-resolution cross-sectional medical images of tissue with a resolution of only a few microns. This technique is commonly used in ophthalmology, but is being studied for dental and dermatological use;
- Light therapy for neurostimulation, which will avoid the malfunction of electrodes that commonly occurs with long-term electrical stimulation; and
- High intensity focused ultrasound, which is a minimally invasive therapy to stop internal bleeding and ablate pathologic tissue.
In the coming months, the Center intends to issue a report highlighting recent achievements in the area of regulatory science and outlining future regulatory science projects and their applicability to medical device development and public health.
In addition, the Center plans to take the following actions to improve regulatory science for medical devices: (1) establish a formal process for CDRH-wide prioritization of scientific research that will include public input; (2) explore establishing public-private partnerships, including at least one new such partnership in 2011 - as we committed to do under our 2011 Strategic Priorities;10 (3) collaborate with other government agencies - for example, we recently executed a Memorandum of Understanding with DARPA allowing us, among other things, to collaborate on developing new tests to quickly and accurately assess the safety and effectiveness of devices; and (4) hold public workshops promoting the advancement of regulatory science - in 2011, CDRH plans to hold at least one public workshop focused on promoting advances in medical device computational modeling and simulation.
As part of the public comment period on CDRH's Innovation Initiative, we are interested in receiving comments about other programs that could support innovation in medical device development by strengthening the research infrastructure in the U.S. and advancing regulatory science.
3. PREPARE FOR AND RESPOND TO TRANSFORMATIVE INNOVATIVE TECHNOLOGIES AND SCIENTIFIC BREAKTHROUGHS
CDRH aims to maintain cutting-edge expertise and experience in-house, but some technologies emerge so rapidly that it is challenging for the Center to be fully prepared in advance for all devices it reviews, and, as a result, delays in review, particularly of the most innovative technologies, can occur. CDRH works to identify and predict developing technologies, and to identify sources of scientific expertise to adequately evaluate those technologies. Under the Innovation Initiative, CDRH would promote the following two programs to enhance our preparedness:
3.1 Horizon Scanning
Medical device technologies emerge and develop rapidly. For example, over the past decade CDRH has experienced significant growth in the need for expertise related to genomics, nanotechnology, biomaterials, and software. Rather than identify and seek out needed expertise when submissions have already been received, CDRH has strategically evaluated its needs through formal horizon scanning and hired or contracted with appropriate experts in advance of receiving device submissions in these areas.
During 2007-2008, CDRH developed a 10-year forecast for medical device technologies designed to help the Center and medical device stakeholders prepare for pioneering products and emerging medical device technologies that will pose new scientific and regulatory challenges.11 The purpose of this forecast was to identify key scientific issues and novel technologies, and to set regulatory, scientific, and administrative strategies that adequately prepare the Center for these developments. Identified emerging areas included specific device technologies (computerized, wireless, robotic), scientific fields (genomic, proteomic, metabolomic, and epigenomic) and use characteristics (geriatric, home use).
CDRH seeks to enhance its horizon scanning methodology, an approach that reviews important scientific literature and accounts for public health needs as well as considers technologies funded by other government agencies, input from manufacturers and other stakeholders with knowledge of the medical device industry, and information from various other sources. Additionally, interval horizon scanning with public reporting of its findings would help CDRH anticipate and prepare for emerging device technologies by focusing our hiring and contracting, staff education, and research efforts in these areas.
3.2 CDRH Network of Experts
Given the need for additional expertise in certain areas to complement the Center's in-house knowledge and experience, CDRH announced its intention to develop a Network (or Networks) of Experts to serve as a resource to assist in better understanding emerging technologies in fields with which our reviewers might not be immediately familiar.12 These Networks will function differently than the Advisory Panels that CDRH currently utilizes for review of certain device submissions. CDRH intends to consult members of the Network on an individual basis for their views on discrete scientific issues and does not intend to seek consensus opinions from more than one Network member as it would do with an advisory committee.13 If the Center finds that it is necessary to disclose proprietary or other privileged information when consulting a Network member, the Center would ensure that any such proprietary or other privileged information is protected in accordance with applicable statutes and regulations. CDRH plans to post an SOP to FDA's website on how the Center will utilize these Networks of Experts by September 15, 2011.14 With the assistance of scientific experts outside the Center, our reviewers can obtain additional perspectives, gain knowledge, and ultimately make better informed decisions, provide clearer expectations for data requirements, and more easily address other scientific issues that arise during the regulatory review process. In addition, engagement with external experts supports a culture of continuous scientific learning, promotes professional staff development, and provides the flexibility needed to review innovative devices in a timely manner.
CONCLUSION
CDRH is committed to assuring that American patients have safe, timely access to important new technologies and next-generation products. CDRH's Innovation Initiative is designed to strengthen the U.S. medical device research infrastructure, promote regulatory science, streamline the conduct of clinical trials, improve the quality and integrity of clinical trial data, identify and prepare for important emerging device technologies, and accelerate the regulatory review of these pioneering products. This program would help CDRH achieve its goal of advancing public health by facilitating innovation to help bring transformative devices to patients and make medical devices safer and more effective.
1 See "510(k) and Science Report Recommendations: Summary and Overview of Comments and Next Steps" and "Plan of Action for Implementation of 510(k) and Science Recommendations."
2 As described in the Expedited Review guidance, see http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/ GuidanceDocuments/ucm089643.htm, FDA is required by statute (section 515(d)(5) of the Federal Food, Drug, and Cosmetic Act) to review only PMAs meeting certain conditions on an expedited basis. FDA, however, uses the criteria as guidelines for expedited review of product development protocols, 510(k)s and de novo classifications.
3 Breakthrough technologies should be demonstrated to lead to a clinical improvement in the treatment or diagnosis of the life-threatening or irreversibly debilitating condition.
4 See Footnote 1. Also see "CDRH Preliminary Internal Evaluations - Volume I: 510(k) Working Group Preliminary Report and Recommendations" and "CDRH Preliminary Internal Evaluations - Volume II: Task Force on the Utilization of Science in Regulatory Decision Making Preliminary Report and Recommendations," both available at http://www.fda.gov/AboutFDA/CentersOffices/ CDRH/CDRHReports/UCM220272.htm.
5 The de novo process was created by the Food and Drug Administration Modernization Act through an amendment to section 513(f)(2) of the Federal Food, Drug, and Cosmetic Act.
6 See Footnote 4.
7 See Footnote 4.
8 See, e.g.; 21 CFR parts 50, 56 and 812.
9 See 21 CFR 814.15.
10 See "CDRH 2011 Strategic Priorities".
11 See "Medical Device Technology Forecast 2008: Future Trends in Medical Device Technologies".
12 See Footnote 1.
13 CDRH does not intend to utilize the network as an advisory committee within the meaning of the Federal Advisory Committee Act, 5 U.S.C. App. 2.
14 See Footnote 1. CDRH plans to address screening of network members for potential conflicts of interest in the SOPs.