Science & Research
-
?
-
Risk Communication
 | The goal of FDA risk communication is to help people make informed decisions about FDA-regulated products. Doing this means conveying both the risks and benefits of these products. It is an interactive process that targets both the public and regulated industries. We do this by |
- communicating directly with product users and prescribers
- giving guidance to industries to help them communicate about their products
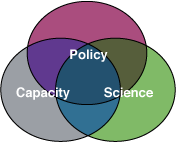
To achieve these goals we aim to strengthen relevant science, policy, and capacity.
Communicating Risks and Benefits: An Evidence-Based User's Guide
?
-
-