Section 2: ART Cycles Using Fresh, Nondonor Eggs or Embryos (Part A)
Section 2 it is broken up into three parts. Part A contains Figures 6–15.
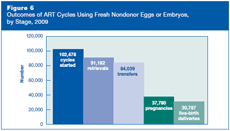
Figure 6 presents the steps for an ART cycle using fresh nondonor eggs or embryos and shows how ART users in 2009 progressed through these stages toward pregnancy and live birth.
An ART cycle is started when a woman begins taking medication to stimulate the ovaries to develop eggs or, if no drugs are given, when the woman begins having her ovaries monitored (using ultrasound or blood tests) for natural egg production.
If eggs are produced, the cycle then progresses to egg retrieval, a surgical procedure in which eggs are collected from a woman’s ovaries.
Once retrieved, eggs are combined with sperm in the laboratory. If fertilization is successful, one or more of the resulting embryos are selected for transfer , most often into a woman’s uterus through the cervix (IVF), but sometimes into the fallopian tubes (GIFT, ZIFT) (see descriptions of ART types).
If one or more of the transferred embryos implant within the woman’s uterus, the cycle then may progress to clinical pregnancy.
Finally, the pregnancy may progress to a live birth, the delivery of one or more live-born infants. (The birth of twins, triplets, or more is counted as one live birth.)
A cycle may be discontinued at any step for specific medical reasons (e.g., no eggs are produced, the embryo transfer was not successful) or by patient choice.
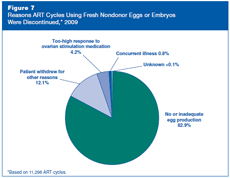
In 2009, 11,296 ART cycles (about 11% out of all 102,478 cycles using fresh nondonor eggs or embryos) were discontinued before the egg retrieval step (see Figure 6). Figure 7 shows reasons that the cycles were discontinued. For approximately 83% of these cycles, there was no or inadequate egg production. Other reasons included too high a response to ovarian stimulation medications (i.e., potential for ovarian hyperstimulation syndrome), concurrent medical illness, or a patient’s personal reasons.
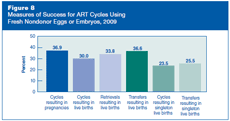
Figure 8 shows ART success rates using six different measures, each providing slightly different information about this complex process. The vast majority of success rates have increased slightly each year since CDC began monitoring them in 1995 (see Section 5).
- Percentage of ART cycles started that produced a pregnancy: This is higher than the percentage of cycles that resulted in a live birth because some pregnancies end in miscarriage, induced abortion, or stillbirth (see Figure 10).
- Percentage of ART cycles started that resulted in a live birth (a delivery of one or more live-born infants):This is the one many people are most interested in because it represents the average chance of having one or more live-born infants by using ART. This is referred to as the basic live birth rate in the Fertility Clinic Success Rate and Certification Act of 1992.
- Percentage of ART cycles in which eggs were retrieved that resulted in a live birth: This is generally higher than the percentage of cycles that resulted in a live birth because it excludes cycles that were canceled before eggs were retrieved. In 2009, about 11% of all cycles using fresh nondonor eggs or embryos were canceled for a variety of reasons (see Figure 7). This is referred to as the live birth rate per successful oocyte (egg) retrieval in the Fertility Clinic Success Rate and Certification Act of 1992.
- Percentage of ART cycles in which an embryo or egg and sperm transfer occurred that resulted in a live birth: This is the highest of these six measures of ART success.
- Percentage of ART cycles started that resulted in a singleton live birth: Overall, singleton live births have a much lower risk than multiple-infant births for adverse infant health outcomes, including prematurity, low birth weight, disability, and death.
- Percentage of ART cycles in which an embryo or egg and sperm transfer occurred that resulted in a singleton live birth: This is higher than the percentage of ART cycles started that resulted in a singleton live birth because not all ART cycles proceed to embryo transfer.
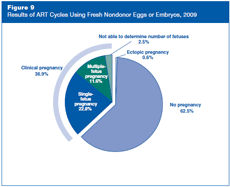
Figure 9 shows the results of ART cycles in 2009 that used fresh nondonor eggs or embryos. Most of these cycles (approximately 63%) did not produce a pregnancy; a very small proportion (less than 1%) resulted in an ectopic pregnancy (the embryo implanted outside the uterus), and about 37% resulted in clinical pregnancy. Clinical pregnancies, accounting for more than one-third of cycles, can be further subdivided as follows:
- Approximately 23% resulted in a single-fetus pregnancy.
- Approximately 12% resulted in a multiple-fetus pregnancy.
- Approximately 3% of pregnancies ended before the number of fetuses could be accurately determined.
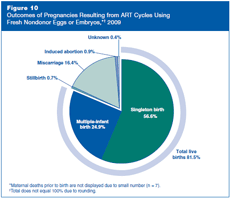
Figure 10 shows the outcomes of pregnancies resulting from ART cycles using fresh nondonor eggs or embryos in 2009. Approximately 82% of the pregnancies resulted in a live birth (about 57% in a singleton birth and 25% in a multiple-infant birth). About 18% of pregnancies resulted in miscarriage, stillbirth, induced abortion, or maternal death prior to birth. For less than 1% of pregnancies, the outcome was unknown.
Although the birth of more than one infant is counted as one live birth, multiple-infant births are presented here as a separate category because they often are associated with problems for both mothers and infants. Infant deaths and birth defects are not included as adverse outcomes because the available information for these outcomes is incomplete.
Multiple-infant births are associated with greater problems for both mothers and infants, including higher rates of caesarean section, prematurity, low birth weight, and infant disability or death.
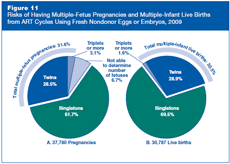
Part A of Figure 11 shows that among the 37,780 pregnancies that resulted from ART cycles using fresh nondonor eggs or embryos, approximately 62% were singleton pregnancies, 29% were twins, and 3% were triplets or more. Approximately 7% of pregnancies ended before the number of fetuses could be accurately determined. Therefore, the percentage of pregnancies with more than one fetus might have been higher than what was reported (about 32%).
In 2009, 6,837 pregnancies resulting from ART cycles ended in either miscarriage, stillbirth, induced abortion, or maternal death, and 156 pregnancy outcomes were not reported. The remaining 30,787 pregnancies resulted in live births. Part B of Figure 11 shows that approximately 31% of these live births produced more than one infant (29% twins and approximately 2% triplets or more). This compares with a multiple-infant birth rate of slightly more than 3% in the general U.S. population.
Although the total rates for multiples were similar between pregnancies and live births, there were more triplet-or-more pregnancies than births. Triplet-or-more pregnancies may be reduced to twins or singletons by the time of birth. This can happen naturally (e.g., fetal death), or a woman and her doctor may decide to reduce the number of fetuses using a procedure called multifetal pregnancy reduction. CDC does not collect information on multifetal pregnancy reductions.
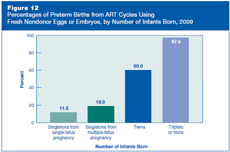
Preterm birth occurs when a woman gives birth before 37 full weeks of pregnancy. Infants born preterm are at greater risk of death in the first few days of life, as well as other adverse health outcomes, including visual and hearing impairments, intellectual and learning disabilities, and behavioral and emotional problems throughout life. Preterm births also cause substantial emotional and economic burdens for families.
Figure 12 shows percentages of preterm births resulting from ART cycles that used fresh nondonor eggs or embryos in 2009, by number of infants born. For singletons, it shows separately the percentage of preterm birth among infants born from pregnancies that started with one fetus (single-fetus pregnancies) and with more than one fetus (multiple-fetus pregnancies).
Among singletons, the percentage of preterm births was higher for those from multiple-fetus pregnancies (19%) than those from single-fetus pregnancies (about 12%). In the general U.S. population, where singletons are almost always the result of a single-fetus pregnancy, 12% were born preterm in 2008 (most recent available data).
Among births resulting from ART cycles that used fresh nondonor eggs or embryos in 2009, 60% of twins and 98% of triplets or more were born preterm. A comparison of preterm births between ART’s multiple-fetus pregnancies and that of the general population is not meaningful because a substantial proportion of twin births or triplet and higher order births are due to infertility treatments (both ART and non-ART).
These data indicate that the risk of preterm birth is higher among infants conceived through ART than for infants in the general population. This increase in risk is due, in large part, to the higher percentage of multiple-fetus pregnancies resulting from ART cycles.
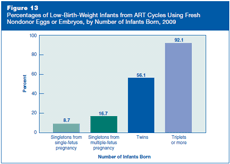
Low-birth-weight infants (less than 2,500 grams, or 5 pounds, 9 ounces) are at increased risk of death and short- and long-term disabilities such as cerebral palsy, intellectual disabilities, and limitations in motor and cognitive skills.
Figure 13 shows percentages of low-birth-weight infants resulting from ART cycles that used fresh nondonor eggs or embryos in 2009, by number of infants born. For singletons, it shows separately the percentage of low birth weight among infants born from pregnancies that started with one fetus (single-fetus pregnancies) and with more than one fetus (multiple-fetus pregnancies).
Among singletons, the percentage of low-birth-weight infants was higher for those from multiple-fetus pregnancies (about 17%) than those from single-fetus pregnancies (about 9%). In the general U.S. population, where singletons are almost always the result of a single-fetus pregnancy, 8% of infants born in 2008 (most recent available data) had low birth weights.
Approximately 56% of twins and 92% of triplets or more resulting from ART cycles in 2009 had low birth weights. Comparing percentages of low birth weights between ART twins and triplets or more and the general population is not meaningful because the vast majority of twin births or triplets and higher order births are due to infertility treatments (both ART and non-ART).
These data indicate that the risk of low birth weight is higher for infants conceived through ART than for infants in the general population. The increase in risk is due, in large part, to the higher percentage of multiple-fetus pregnancies resulting from ART cycles.
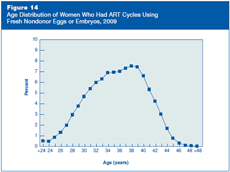
Figure 14 presents ART cycles using fresh nondonor eggs or embryos in 2009 according to the age of the woman who had the procedure. About 12% of these cycles were among women younger than age 30, about 66% were among women aged 30–39, and approximately 22% were among women aged 40 or older.
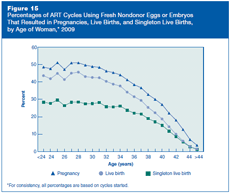
Do percentages of ART cycles that result in pregnancies, live births, and singleton live births differ among women of different ages?
A woman’s age is the most important factor affecting the chances of a live birth when her own eggs are used. Figure 15 shows percentages of pregnancies, live births, and singleton live births among women of different ages who had ART procedures using fresh nondonor eggs or embryos in 2009. Percentages of ART cycles resulting in live births and singleton live births are different because of the high percentage of multiple-infant deliveries counted among the total live births. The percentage of multiple-infant births is particularly high among women younger than 35 (see Figure 36). Among women in their 20s, percentages of ART cycles resulting in pregnancies, live births, and singleton live births were relatively stable; however, percentages declined steadily from among women in their mid-30s onward. For additional detail on percentages of ART cycles that resulted in pregnancies, live births, and singleton live births among women aged 40 or older, see Figure 16.
Read next sections:
Section 1 | Section 2A | Section 2B | Section 2C | Section 3 | Section 4 | Section 5
Contact Us:
- Centers for Disease Control and Prevention
1600 Clifton Rd
Atlanta, GA 30333 - 800-CDC-INFO
(800-232-4636)
TTY: (888) 232-6348 - New Hours of Operation
8am-8pm ET/Monday-Friday
Closed Holidays - cdcinfo@cdc.gov