About FDA
ORA Offices & Divisions
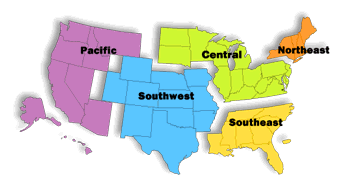
ORA staff are dispersed throughout the United States. Over 85 percent of ORA’s staff works in 5 Regional Offices, 20 District Offices, 13 Laboratories, and more than 150 Resident Posts and Border Stations. ORA Headquarters is comprised of the Office of Resource Management; Office of Regional Operations; and, the Office of Enforcement located in Rockville, Maryland and the Office of Criminal Investigations located throughout the U.S. FDA maintains offices and staff in Washington, D.C., the U.S. Virgin Islands, Puerto Rico, and in all States except Wyoming.
The duties and functions of the Office and components of ORA can be viewed by clicking on the component name in the list below. The offices that report directly to ORA are shown here in UPPER CASE. Divisions within each office are in lower case.
Division of Management Operations
Division of Field Investigations
OFFICE OF CRIMINAL INVESTIGATIONS
REGIONAL & DISTRICT OFFICE CONTACTS