International Programs
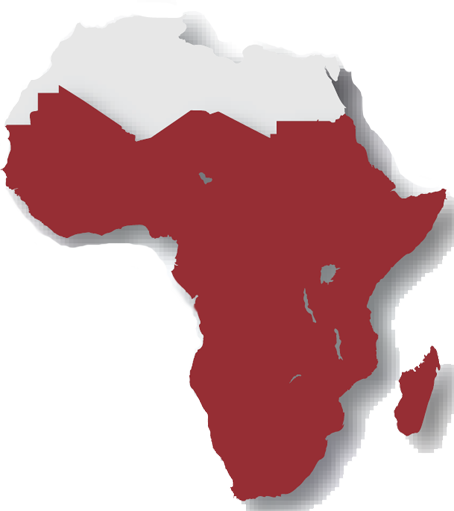
Sub-Saharan Africa
FDA’s activities ininclude engaging with foreign regulatory counterparts on all products over which FDA has regulatory responsibility. This includes managing the sharing of non-public information and addressing regulatory compliance issues, some of which have a global impact. FDA’s activities and sharing of technical expertise with its foreign regulatory counterparts enhances a country’s ability to produce safe and effective foods and medical products and provide exports that meet the FDA standard. Information sharing and capacity building activities help the foreign country to enhance its regulatory infrastructure, safeguard the health of its citizens and contribute to global health. Also, the FDA activities ensure that constructive coordination of technical assistance results from specific arrangements with countries in Sub-Saharan Africa with regional economic communities such as SADC (Southern Africa Development Community).
Contact FDA
U.S. Food and Drug Administration
Office of International Programs
Sub-Saharan Africa
U.S. Embassy
877 Pretorius Street
Pretoria, South Africa 0001