Safety
Goals Are To Maximize Benefit, Minimize Risk
Table of Contents: Managing the Risks From Medical Product Use: Creating a Risk Management Framework
Previous Section : Part 1 Background - What are the Risks and what is FDA"s role in Managing Risk?
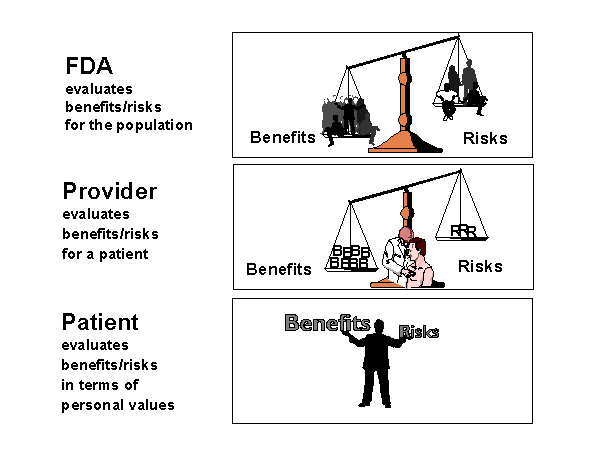
The choice to use a drug, biological product, or device involves balancing the benefits to be gained with the potential risks of using a product. As illustrated above, an elaborate system has developed in the United States with the goals of maximizing the benefits and minimizing the risks associated with using medical products. Under this system, medical products must undergo FDA approval before marketing. FDA's premarketing review involves (1) developing criteria for the evidence of product safety and effectiveness that manufacturers must submit to FDA, and (2) evaluating the data manufacturers submit to see if the product meets the statutory standard for market approval. Briefly, the system works as follows. After a systematic development process that includes clinical trials, the manufacturer submits an application to the FDA for approval. After a thorough review of the data, FDA makes a decision to approve or not approve a product to treat a specific condition, based on a benefit-risk analysis for the intended population and use. Although medical products are required to be safe, safety does not mean zero risk, since all medical products are associated with risks. A safe medical product is one that has reasonable risks, given the magnitude of the benefit expected and the alternatives available.
One result of FDA's premarketing evaluation of a new product is the approval of its labeling. The labeling must indicate which patients are appropriate for treatment, identify the product's potential adverse side effects, and explain how the product should be used to maximize benefits and minimize adverse side effects.
Once approved, products move swiftly into the marketplace for use by prescribers and patients. As shown in the next figure on balancing risks and benefits, after FDA evaluates the risks and benefits for the population, the prescriber is central to managing risks and benefits for the individual. In addition, patients make decisions about treatment choices based on their personal valuation of benefits and risks. In the context of an individual treatment decision, FDA's role in reducing risk involves ensuring that accurate, substantiated, and balanced information about a product is available to the prescriber and the patient. This system, when functioning well, succeeds in managing a balance between benefit and risk. But FDA's mission to ensure the safety of medical products cannot be accomplished without effective partnerships with healthcare practitioners and the public.
FDA also operates postmarketing surveillance programs intended to identify unexpected risks of approved products. When new risks are identified in a medical product, the manufacturer adds them to the labeling, or, if serious enough, they may trigger an Agency reevaluation of the approval decision. Recent concerns about the safety of medical products have focused on several types of risks. For newly approved products, concerns have centered on unanticipated side effects that emerge after a product is on the market. In addition, concerns have been raised about FDA's ability to ensure the appropriate use of regulated products in medical practice. For example, how far should the Agency intrude into traditional areas of medical practice when the safe use of a product requires practitioner training, or frequent patient blood testing? Is the Agency responsible when a medical product is used beyond the parameters of the approved labeling? Some reports have focused on the human and economic costs of medication errors, while others are concerned about serious adverse events that have occurred even when a medical product has been used appropriately. Because each of these types of risks has a different source, effective management of each is likely to be different. To understand the complexity of managing the risks associated with using medical products, it is important to understand the different types of risks and their sources.
Next Section : What are the Risks involved with using Medical Products?