Medical Devices
Color Additives For Medical Devices
INTRODUCTION
Under certain conditions, color additives in medical devices are subject to the same provisions that apply to color additives in foods, drugs and cosmetics. The Food, Drug and Cosmetic (FD&C) Act states that devices containing a color additive are considered unsafe, and thereby adulterated, unless a regulation is in effect listing the color additive for such use. The FD&C Act limits applicability of these provisions to color additives that directly contact the body for a significant period of time. At the present time, the term a "significant period of time" is not defined by FDA regulation.
For the purposes of enforcing adulteration provisions under the FD&C Act, unless the color additive and its use conform to a listing regulation under Title 21 of the Code of Federal Regulations (CFR) Parts 73 and 74, including any provision of such regulation prescribing the conditions under which the color additive may be safely used. The color listing regulation may permit use of the color additive in a generic type of device, such as contact lenses, or may place limitations on its use, such as polypropylene nonabsorbable sutures for general surgical use but not for ophthalmic surgical use.
A color additive subject to the provisions under section 721 of the FD&C Act is deemed unsafe The 21 CFR Parts 1 to 99 (includes Parts 70,71,73,74 and 80) may be obtained from U.S. Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402, Phone # 202-512-1800. The other documents mentioned herein may be ordered from FDA, Division of Small Manufacturers Assistance, International and Consumer Assistance (DSMICA), 10903 New Hampshire Avenue, WO66 Room 4621, Silver Spring, MD 20993. Phone (301) 796-7100 or (800) 638-2041, FAX (301) 847-8149. You may send an e-mail request to dsmica@fda.hhs.gov.
COLOR ADDITIVE DEFINED
A) is a dye, pigment, or other substance made by a process of synthesis or similar artifice, or extracted, isolated, or otherwise derived, with or without intermediate or final change of identity, from a vegetable, animal, mineral, or other source, and
B) when added or applied to a food, drug, or cosmetic, or to the human body or any part thereof, is capable (alone or through reaction with other substance) of imparting color thereto; except that such term does not include any material which the Secretary [of the Department of Health and Human Services] by regulation, determines is used (or intended to be used) solely for a purpose or purposes other than coloring.
2) The term "color" includes black, white and intermediate grays.
COLOR ADDITIVE GENERAL PROVISIONS
Significant provisions of the color additive regulations under Title 21 of the CFR will be found under the following parts:
70.3 Definitions
70.5 General restrictions on use of color additives
(a) Color additives for use in the area of the eye
(c) Color additives for use in surgical sutures
70.25 Labeling requirements for color additives (other than hair dyes).
70.40 Safety factors to be considered
70.42 Criteria for evaluating the safety of color additives
71 Color additive petitions
73 Listing of color additives exempt from certification
74 Listing of color additives subject to certification
80 Color additive certification
Subpart A - General provisions
Subpart B - Certification procedures
CERTIFICATION
Before entering commerce, a color additive listed in 21 CFR Part 74 must be submitted by the manufacturer to the FDA, Center for Food Safety and Applied Nutrition, Office of Cosmetics and Colors, Color Certification Branch, for testing to make certain the color additive meets FDA specifications. Failure of the color additive to meet specifications, as described in Part 74, will result in a statement of refusal to enter commerce issued by the FDA.
EXEMPT FROM CERTIFICATION
Color additives exempt from certification by FDA are found in 21 CFR Part 73. For exempt additives, testing may be done by the color additive manufacturer to make certain the color additives meet FDA specifications.
The medical device manufacturer that intends to use the color additive should obtain appropriate information from the color additive manufacturer to show that the color additive is suitable for the intended use. Of course the finished device manufacturer may have to do additional evaluation of the color in the finished device.
INVESTIGATIONAL DEVICE EXEMPTIONS REGARDING COLOR ADDITIVES
The investigational device exemption (IDE) regulations 21 CFR Part 812 exempt from the requirements of section 721 a color additive or any specific use of the color additive when intended solely for investigational use by qualified experts and when such exemption is consistent with the need needs of public health.
PETITIONS FOR LISTING OF COLOR ADDITIVES
Petitions for the listing of a color additive, referenced in 21 CFR Part 71, in or on a medical device have typically been filed with FDA by the device manufacturer, rather than the color additive manufacturer, as there is usually not a financial incentive for the color additive manufacturer to file.
GENERAL RESTRICTIONS ON USE OF COLOR ADDITIVES
Color additives have general restrictions under 21 CFR Part 70.5, when used on or in a surgical suture and/or for use in the area of the eye. A color additive must be specifically certified or listed by FDA under Parts 73, 74, and 81, for use in or on a surgical suture or for use in the area of the eye, to be used for these purposes. A listing or certification for other uses does not allow the color additive to be used in or on a surgical suture or in the area of the eye, nor to be considered listed or certified for these uses under Parts 73, 74 and 81.
INTRODUCTION to 21 CFR PARTS 73 & 74
The color additive common names, chemical names, use restrictions and specific limitations are found in 21 CFR Parts 73 and 74. Common names used in place of chemical names are from the "Color Index (CI) International," Volume 8, 1987. Consult 21 CFR Parts 73 and 74 for additional information on the proper combination of color additives, percent by weight and restrictions of use.
The 21 CFR Parts 73 and 74, Subpart D, identified color additives for use in medical devices.
They are as follows:
| # of Colors | Medical Device Use |
|---|---|
| 36 | Contact lenses |
| 21 | Sutures in general surgery |
| 13 | Sutures in ophthalmic surgery |
| 03 | Intraocular lenses, for supporting haptics |
| 01 | Bone cement |
| 01 | Soft(hydrophilic) contact lens, to mark r or l for identification (r or l is right or left). |
PART 73 - COLOR ADDITIVES EXEMPT FROM CERTIFICATION, TABLE
Certification of the color additives in Part 73 is not necessary for the protection of the public health; and, therefore, the following batches are exempt from the certification requirement of 721(c) of the FD&C Act. The following tables are from 21 CFR Parts 73 and 74, Subpart D. The acronym C.I., as referenced in these tables, stands for Acolor index@.
| 21 CFR | COLOR ADDITIVE | USES AND RESTRICTIONS | |
|---|---|---|---|
| 73.1015 | Chromium-Cobalt- Aluminum Oxide (Blue green pigment) | For coloring linear polyethylene surgical sutures for use in general surgery. | |
| 73.1025 | Ferric Ammonium Citrate | May be used in combination with pyro-(green or brown)gallol, as listed in 73.1375, for coloring plain or chromic catgut sutures for use in general and ophthalmic surgery. | |
| 73.1375 | Pyrogallol C.I.Oxidation Base 32 | May be used in combination with ferric ammonium citrate, as listed in 73.1025, for coloring plain and chromic catgut sutures for use in general and ophthalmic surgery. | |
| 73.1410 | Logwood Extract C.I. Natural Black 1 (redish brown to black) | For coloring nylon 66, nylon 6 or silk non-absorbable sutures for use in general and ophthalmic surgery. | |
| 73.3105 | C.I. Solvent blue 101 Chemical name: 1,4-Bis[(2 methylphenyl) amino]-9,10-anthracenedione. | Contact lenses | |
| 73.3106 | C.I. Reactive Blue 246 Chemical name: 1,4-bis[4-[(2-methacryl- oxyethyl)phenylamino] anthraquinone. | Contact lenses | |
| 73.3107 | Carbazole violet C.I. Pigment violet 23 | Contact lenses | |
| 73.3110 | Chlorophyllin-copper (Green) | For coloring polymethylmethacrylate bone cement. | |
| 73.3110a | Chromium-cobalt- aluminum oxide C.I. Pigment blue 36 | Contact lenses | |
| 73.3111 | Chromium oxide greens | Contact lenses | |
| 73.3112 | C.I. Vat orange 1 | Contact lenses | |
| 73.3115 | 2-[[2,5-Diethoxy-4- [(4-methylphenyl)thiol] phenyl]azo]-1,3,5- benzenetriol (common name not available). | May be used to mark soft (hydrophilic) contact lenses with r or l for right and left identification. | |
| 73.3117 | C.I. Vat brown 1 Chemical name: 16,23-Dihydrodinaphtho [2,3-a:2',3'-I] napth [2', 3':6,7] indolo [2,3-c] carbazole-5,10,15,17,22,24-hexone. | Contact lenses | |
| 73.3118 | C.I. Vat yellow 3 Chemical name: N,N'-(9,10-Dihydro-9,10 dioxo-1,5-anthracenediyl) bisbenzamide | Contact lenses | |
| 73.3119 | C.I. Vat blue 6 Chemical name: 7,16-Dichloro-6,15- dihydro-5,9,14,18- anthrazinetetrone | Contact lenses | |
| 73.3120 | C.I. Vat green 1 Chemical name: 16,17-Dimethoxydinaphtho [1,2,3-cd:3',2',1'-lm] perylene-5,10-dione. | Contact lenses | |
| 73.3121 | Poly(hydroxyethyl meth- acrylate)-dye copolymers | Contact lenses | |
| The color additives are formed by reacting one or more of the reactive dyes listed in 73.3121 with poly(hydroxyethyl methacry-late). The dyes that may be used alone or in combination are: | |||
| (1) C.I. Reactive black 5 (2) C.I. Reactive blue 21 (3) C.I. Reactive orange 78 (4) C.I. Reactive yellow 15 (5) C.I. Reactive blue 19 (6) C.I. Reactive blue 4 (7) C.I. Reactive red 11 (8) C.I. Reactive yellow 86 (9) C.I. Reactive blue 163 (10) C.I. Reactive red 180 | |||
| 73.3122 | C.I. Solvent yellow 18 Chemical name: 4-[(2,4-dimethylphenyl) azo]-2,4-dihydro-5-methyl -2-phenyl-3H-pyrazol-3-one | Contact lenses | |
| 73.3123 | C.I. Vat orange 5 Chemical name: 6-Ethoxy-2-(6-ethoxy-3- oxobenzo[b]thien-2(3H)- ylidene)benzo[b]thiophen -3 (2H)-one. | Contact lenses | |
| 73.3124 | Phthalocyanine green C.I. Pigment green 7 | Contact lenses | |
| 73.3125 | Iron oxides (Red) | Contact lenses | |
| 73.3126 | Titanium dioxide (White) | Contact lenses | |
| 73.3127 | Vinyl alcohol/methyl methacrylate-dye react products. | Contact lenses | |
The color additives are formed by reacting the dyes, either alone or in combination, with a vinyl alcohol/methyl methacrylate copolymer.
The dyes are:
(1) C.I. Reactive Red 180
(2) C.I. Reactive Black 5
(3) C.I. Reactive Orange 78
(4) C.I. Reactive Yellow 15
(5) C.I. Reactive Blue 19
(6) C.I. Reactive Blue 21
PART 74 - COLOR ADDITIVES SUBJECT TO CERTIFICATION
All batches of color additives subject to certification, shall be certified in accordance with regulations in 21 CFR part 80 of this chapter.
| 21 CFR | COLOR ADDITIVE | USES AND RESTRICTIONS |
|---|---|---|
| 74.1102 | FD&C Blue No. 2 | For coloring nylon surgical sutures for use in general surgery. |
| 74.1109 | D&C Blue No. 9 | For coloring cotton and silk surgical sutures, including sutures for opthalmic use. |
| 74.1205 | D&C Green No. 5 | For coloring nylon 66 and or nylon 6 nonabsorbable surgical sutures for use in general surgery. |
| 74.3045 | [Phthalocyaninato(2-)] | For coloring: |
| Copper | Polypropylene sutures | |
| Polybutester nonabsorbable sutures for use in general and ophthalmic surgery. | ||
| Polymethyl methacrylate monofilament used as supporting haptics for intraocular lenses. | ||
| Polybutylene terephthalate nonabsorbable | ||
| monofilament sutures for general and ophthalmic surgery. | ||
| Contact lenses | ||
| 74.3106 | D&C Blue No. 6 | For coloring: |
| Polyethylene terephthalate surgical sutures for general surgical use. | ||
| Plain or chromic collagen absorbable sutures for ophthalmic surgical use and general surgical use. | ||
| Polypropylene surgical sutures for general surgical use. | ||
| Polydioxanone synthetic absorbable sutures for ophthalmic and general surgical use. | ||
| 74.3206 | D&C Green No. 6 | For coloring: |
| Contact lenses | ||
| Polyethylene terephthalate surgical sutures, including sutures for ophthalmic use. | ||
| Polyglycolic acid surgical sutures and sutures for ophthalmic use. | ||
| Poly(glycolic acid-co-trimethylene carbonate) sutures for general surgical use. | ||
| Polymethylmethacrylate support haptics of intraocular lenses. | ||
| 74.3230 | D&C Red No. 17 | Contact lenses |
| 74.3602 | D&C Violet No. 2 | For coloring: |
| Polyglactin 910 (glycolic-lactic acid polyester) synthetic absorbable sutures for use in general and ophthalmic surgery. | ||
| Polydioxanone synthetic absorbable sutures for use in general and ophthalmic surgery. | ||
| Polymethylmethacrylate intraocular lens haptics. | ||
| Contact lenses | ||
| Polyglecaprone 25 (epsilon-caprolactone/glykcolide copolymer)synthetic absorbable sutures for use in general surgery. | ||
| Poly (epsilon-caprolactone) absorbable sutures for use in general surgery. | ||
| 74.3710 | D&C Yellow No. 10 Contact lenses |
CHEMISTRY GUIDANCE FOR LISTING COLOR ADDITIVES IN CONTACT LENSES*
- Permanently listed color additives, as referenced in 21 CFR Parts 73 & 74, approved for eye use in cosmetics, will be approved by CDRH for use as lens colors, with no further testing required.
- 1) Permanently listed color additives not approved for eye area use and
(2) Other color additives intended as colors in contact lenses, will be evaluated on a case-by-case basis. Testing requirements will be dependent on the exposure levels and the degree of concern for potential toxicity of the color additive and its contaminants. Chemistry and available toxicology information should be in the submission, as it will be considered in the determination of safety.
- A color additive which has been demonstrated to be a carcinogen will not be approved.
- Carcinogenic contaminants (1)in color additives which have been found to be negative in a carcinogenicity bioassay or (2) in color additives which have not been tested in a bioassay, will be subject to risk assessment. If the lifetime risk of cancer, based on a worst-case exposure estimate, is considered to be sufficiently low, use of the color additive may be approved.
- The in vivo Three Week Ocular Irritation Test in Rabbits and the Guinea Pig Sensitization Test, as described in the ATOXICOLOGY@ section, should be conducted with both colored and uncolored lenses
- A cytotoxicity test on the color additive (neat**) and on extracts*** of colored and uncolored lenses should be performed. This test should allow direct exposure of the cells to the tested color additive or lens extract to provide dose response information; the agar overlay test is therefore not recommended for this purpose. A 28 day repeated instillation ocular irritancy test can be substituted for a cytotoxicity test.
*Reprinted from: PREMARKET NOTIFICATION (510(K)) GUIDANCE DOCUMENT FOR DAILY WEAR CONTACT LENSES, REVISED MAY 1994. Edited for use in this document.
**Neat is defined as undiluted
***For extraction procedures, see the Toxicology Section of the PREMARKET NOTIFICATION (510(K)) GUIDANCE DOCUMENT FOR DAILY WEAR CONTACT LENSES.
The following is a flow chart diagramming the major steps and requirements for safety review by the FDA, Division of Food and Color Additives, of color additives in contact lenses.
AVAILABLE INFORMATION
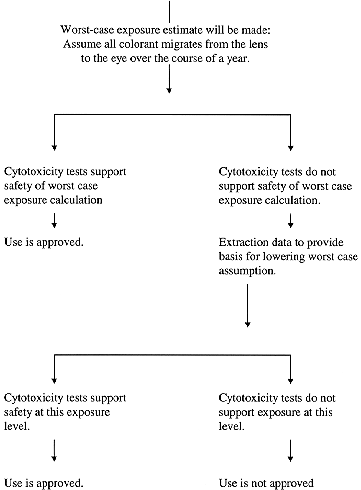
Note: A 28-day repeated installation ocular irritancy test with the neat dye and with lens extracts can be substituted for cytotoxicity tests.
COLOR ADDITIVE PETITIONS*
A color additive for use in or on a medical device is subject to the requirements of section 721 of the Federal Food, Drug, and Cosmetic Act (the Act) if the color additive comes in direct contact with the human body for a significant period of time. Because the Medical Device Amendments of 1976 lack guidance in determining whether there is direct contact for a significant period of time, the applicant should contact the Premarket Approval (PMA) Staff, WO66-1650, Center for Devices and Radiological Health, Food and Drug Administration, 10903 New Hampshire Avenue, Silver Spring, MD 20993, for assistance in determining whether the use of the color additive is subject to the requirements of section 721. FDA has determined that color additives used in tinted soft contact lenses, dyed absorbable and nonabsorbable surgical sutures, and bone cements are subject to the requirements of section 721.
The term "color additive" means a material which (1) is a dye, pigment, or other substance made by a process of synthesis or similar artifice, or extracted, isolated or otherwise derived, with or without intermediate or final change of identity from a vegetable, animal, mineral, or other source; and (2) when added or applied to a [medical device] or to the human body or any part thereof is capable (alone or through reaction with other substance) of imparting color thereto. The term "color additive" does not include any material which FDA determines is used (or intended to be used) solely for a purpose or purposes other than coloring.
A color additive subject to section 721 of the Act is considered unsafe and thereby adulterating the device within the meaning of section 501(a)(4) of the Act unless the color additive is listed for the appropriate use in accordance with section 721 of the Act. The listing regulation may permit unqualified use of the color additive in a generic type of device such as contact lenses or may place various limitations on its use such as polypropylene nonabsorbable sutures for general but not ophthalmic surgical use. The color additive must be from a batch certified in accordance with regulations issued under section 721 (c) for that use, unless the color additive has been exempted from the certification requirement. The investigational device exemption (IDE) regulations 21 CFR Part 812 include provisions for exempting from the requirements of section 721 a color additive or any specific use of the color additive when intended solely for investigational use by qualified experts and when such exemption is consistent with the public health.
If a color additive subject to section 721 is used in or on a device subject to premarket approval and it has not previously been listed for such use, in lieu of submitting a color additive petition (CAP) under 21 CFR 71, the applicant may submit the information required under Part 71 as part of the PMA. When submitted as part of the PMA as provided by 21 CFR 814.20(f), the information must be submitted in three copies, each bound in one or more numbered volumes of reasonable size.
The applicant is cautioned that a PMA for a device that contains a color additive subject to listing under section 721 will not be approved by FDA until the color additive is listed for use in or on the device. Regulations listing color additives for use in medical devices are codified under Subpart D of 21 CFR Part 73 if exempt from certification and under Subpart D of 21 CFR Part 74 if subject to certification.
To assist in developing an appropriate strategy for submitting a color additive petition, the PMA applicant is advised to consider the following:
- The review period for a color additive petition may be as long as, and may extend beyond, that for a PMA.
- A color additive petition included in a PMA will be processed in accordance with the procedures and fee schedules in 21 CFR 71.
- Because all color additive petitions are processed by the FDA Center for Food Safety and Applied Nutrition (CFSAN), processing of the color additive petition will be delayed if it cross references rather than includes required information in the remaining portion of the PMA reviewed by CDRH.
- The existence of a color additive petition in a PMA should be highlighted in the PMA cover letter to minimize delays in forwarding the color additive petition by CDRH to CFSAN for processing
- A color additive petition included in a PMA is subject to the confidentiality provisions of 21 CFR Part 71 rather than those in 21 CFR Part 814.
*Reprinted from Premarket Approval (PMA) Manual, FDA 87-4214. Edited for use in this document.
FDA CONTACT POINTS FOR COLOR ADDITIVES
Center for Devices and Radiological Health
Nicole Wolanski
FDA Center for Devices and Radiological Health
Office of Device Evaluation
Premarket Approval (PMA) Staff
10903 New Hampshire Avenue, WO66-1650
Silver Spring, Maryland 20993
301-796-5640
General Information and Publications
Walter Snesko
FDA Center for Devices and Radiological Health
Office of Communication, Education, and Radiation Programs
Division of Small Manufacturers, International and Consumer Assistance
10903 New Hampshire Avenue, WO66-4621
Silver Spring, Maryland 20993
301-796-7100, or 1-800-638-2041, FAX 301-847-8149
APPENDIX
PART 80 COLOR ADDITIVE CERTIFICATION
Subpart A - General Provisions
Sec.
80.10 Fees for certification services.
Subpart B - Certification Procedures
80.21 Request for certification.
80.22 Samples to accompany requests for certification.
80.31 Certification.
80.32 Limitations of certificates.
80.34 Authority to refuse certification service.
80.35 Color additive mixtures; certification and exemption from certification.
80.37 Treatment of batch pending certification.
80.38 Treatment of batch after certification.
80.39 Records of distribution.
SUBPART A - GENERAL PROVISIONS
80.10 Fees for certification services.
(a) Fees for straight colors including lakes. The fee for the services provided by the regulations in this part in the case of each request for certification submitted in accordance with sec. 80.21(j)(1) and (j)(2) shall be $0.35 per pound of the batch covered by such requests, but no such fee shall be less than $224.
(b) Fees for repacks of certified color additives and color additive mixtures. The fees for the services provided under the regulations in this part in the case of each request for certification submitted in accordance with 80.21(j)(3) and (j)(4) shall be:
(1) 100 pounds or less--$35.
(2) Over 100 pounds but not over 1,000 pounds--$35 plus $0.06 for each pound over 100 pounds.
(3) Over 1,000 pounds--$89 plus $0.02 for each pound over 1,000 pounds.
(c) Advance deposits. Any person regularly requesting certification services may deposit funds in advance of requests as prepayment of fees required by this section.
(d) Method of payment. All deposits and fees required by this section shall be paid by money order, bank draft, or certified check, drawn to the order of the Food and Drug Administration, collectable at par at Washington, DC. All such deposits and fees shall be forwarded to the Center for Food Safety and Applied Nutrition (HFS-100), Food and Drug Administration, 5100 Paint Branch Pkwy., College Park, MD 20740, whereupon after making appropriate records thereof, they will be transmitted to the Treasurer of the United States for deposit to the special account "Salaries and Expenses, Certification, Inspection, and Other Services, Food and Drug Administration."
(e) Refunds from advance deposits. Whenever in the judgment of the Commissioner the ratio between fees collected (which are based upon experience and the best estimate of costs and the best estimate of earnings) and the costs of providing the service during an elapsed period of time, in the light of all circumstances and contingencies, warrants a refund from the fund collected during such period, he shall make ratable refunds to those persons to whom the services were rendered and charged, except that no refund shall be made where the computed ratable amount for the elapsed period is less than $5.00.
SUBPART B - CERTIFICATION PROCEDURES
80.21 Request for certification.
A request for certification of a batch of color additive shall:
(a) Be addressed to the Commissioner of Food and Drugs.
(b) Be prepared in the manner set forth in paragraph (j) of this section.
c) Be submitted in duplicate.
(d) Be signed by a responsible officer of the person requesting certification of the batch. In the case of a foreign manufacturer, the request for certification must be signed by a responsible officer of such firm, and, by his agent who resides in the United States.
(e) Show the name and post office address of the actual manufacturer incase such manufacturer is not the person requesting certification of the batch.
(f) Be accompanied by the fee prescribed in sec. 80.10 unless the person has established with the Food and Drug Administration an advanced deposit to be used for prepayment of such fees. In no case shall the Commissioner consider a request for certification of a batch of color additive if the fee accompanying such request is less than that required by sec. 80.10 or if such fee exceeds the amount held in the advance deposit account of the manufacturer submitting such request for certification.
(g) Be accompanied by the sample prescribed in sec. 80.22 consisting of:
(2) Two ounces in the case of repacks and mixtures.
A sample accompanying a request for certification must be submitted under separate cover and should be addressed to the Color Certification Branch.
(h) The name of a color additive shall be given in the following manner:
(1) The name of a straight color shall be the name of the color as listed in parts 74 and 81 of this chapter.
(2) The name of a lake shall be the name derived in the manner described in part 82 of this chapter.
(3) The name of a mixture shall be the name given to such mixture by the person requesting certification.
(4) The name of a repack shall be the name described in paragraph (h)(1), (2), or (3) of this section, whichever is applicable.
(i) The information and samples enumerated in paragraphs (a) to (h), inclusive, of this section are the minimum required. Additional information and samples shall be submitted at the request of the Food and Drug Administration when such additional information and samples are necessary to determine compliance with the requirements of sec. 80.31 for the issuance of a certificate.
(j) The form for submission of the application shall be one of the following, depending upon whether the color additive is a straight color, a lake, a repack of a previously certified color additive, or a color additive mixture.
(1) Request for certification of a batch of straight color additive.
Office of Cosmetics and Colors (HFS-100),
Center for Food Safety and Applied Nutrition,
Food and Drug Administration,
5100 Paint Branch Pkwy.,
College Park, MD 20740
In accordance with the regulations promulgated under the Federal Food, Drug, and Cosmetic Act, we hereby make application for the certification of a batch of straight color additive.
| Name of color | ________________________ | Batch number | ________________________ |
| (As listed in 21 CFR part 74) | (Manufacturer's number) |
Batch weighs______________ pounds
Batch manufactured by_________________________at________________________________
(Name and address of actual manufacturer)
How stored pending certification___________________________________________________
_____________________________________________________________________________
(State conditions of storage, with kind and size of containers, location, etc.)
Certification requested of this color for use in_________________________________________
_____________________________________________________________________________
_____________________________________________________________________________
Required fee, $_________________ (drawn to the order of Food and Drug Administration).
The accompanying sample was taken after the batch was mixed in accordance with 21 CFR 80.22 and is accurately representative thereof.
| (Signed)_________________________________ | |
| By______________________________________ | |
| (Title) |
2) Request for certification of a batch of color additive lake.
Office of Cosmetics and Colors (HFS-100),
Center for Food Safety and Applied Nutrition,
Food and Drug Administration,
5100 Paint Branch Pkwy.,
College Park, MD 20740
In accordance with the regulations promulgated under the Federal Food, Drug, and Cosmetic Act, we hereby make application for the certification of a batch of color additive lake.
| Name of color | ________________________ | Batch number | ___________________________ |
| (Manufacturer's number) |
Batch weighs________________ pounds.
Name of color used______________________________ Quantity_______________ pounds
Lot number______________________
(When certification of the lake for use in foods is requested)
Precipitant used__________________________
Substratum used_________________________ Quantity___________________ pounds
Batch manufactured by_________________________ at________________________________
(Name and address of actual manufacturer)
How stored pending certification___________________________________________________
_____________________________________________________________________________
(State conditions of storage, with kind and size of containers, location, etc.)
Certification requested of this color for use in_________________________________________
_____________________________________________________________________________
Required fee, $__________(drawn to the order of Food and Drug Administration).
The accompanying sample was taken after the batch was mixed in accordance with 21 CFR 80.22 and is accurately representative thereof.
| (Signed)_________________________________ | |
| By______________________________________ | |
| (Title) |
(3) Request for certification of a repack of a batch of certified color additive.
Date____________________
Office of Cosmetics and Colors (HFS-100),
Center for Food Safety and Applied Nutrition,
Food and Drug Administration,
5100 Paint Branch Pkwy.,
College Park, MD 20740
In accordance with the regulations promulgated under the Federal Food, Drug, and Cosmetic Act, we hereby make application for the certification of a batch of color additive repack.
Name of color______________________________
(As listed in regulations and as certified; or repacker's name, if a mixture)
Original lot number__________________Certified color content__________________________
This color obtained from__________________________Batch number_____________________
Batch weighs_______________pounds
How stored pending certification___________________________________________________
______________________________________________________________________________
(State conditions of storage, with kind and size of containers, location, etc.)
Certification requested for use in___________________________________________________
______________________________________________________________________________
Required fee, $__________(drawn to the order of Food and Drug Administration).
The accompanying sample was taken after the batch was mixed in accordance with 21 CFR 80.22 and is accurately representative thereof.
| (Signed)_________________________________ | |
| By______________________________________ | |
| (Title) |
(4) Request for certification of a batch of color additive mixture.
Date_______________________________
Office of Cosmetics and Colors (HFS-100),
Center for Food Safety and Applied Nutrition,
Food and Drug Administration,
5100 Paint Branch Pkwy.,
College Park, MD 20740
In accordance with the regulations promulgated under the Federal Food, Drug, and Cosmetic Act, we hereby make application for the certification of a batch of color additive mixture.
| Name of mixture | ________________________ | Batch number | ______________________ |
| (Manufacturer's trade name) | (Manufacturer's number) |
Weight of batch__________pounds. Volume of batch__________(If liquid) gallons
Batch manufactured by____________________________________
Constituents of the mixture:
1. Color(s). (List separately each color and each lot number.)
| Name of color as certified | Lot number |
____________________________________________________________________________
____________________________________________________________________________
| Quantity used (in pounds) | Obtained from |
____________________________________________________________________________
____________________________________________________________________________
2. List of diluents. (List separately each diluent.)
Name of diluent
____________________________________________________________________________
____________________________________________________________________________
Quantity used
| By weight | By volume (if liquid) |
____________________________________________________________________________
____________________________________________________________________________
Batch mixed as follows__________________________________________________________
___________________________________________________________________________
(Describe in detail)
How stored pending certification__________________________________________________
____________________________________________________________________________
(State conditions of storage, with kind and size of containers, location, etc.)
Certification requested for use in___________________________________________________
_____________________________________________________________________________
(State proposed uses)
Required fee, $__________(drawn to the order of Food and Drug Administration).
The accompanying sample was taken after the batch was mixed in accordance with 21 CFR 80.22 and is accurately representative there of.
| (Signed)_________________________________ | |
| By______________________________________ | |
| (Title) |
80.22 Samples to accompany requests for certification.
A sample of a batch of color additive which is to accompany a request for certification shall:
(a) Be taken only after such batch has been so thoroughly mixed as to be of uniform composition throughout.
(b) Held under the control of the person requesting certification until certified.
(c) Be labeled to show:
(2) The manufacturer's batch number.
(3) The quantity of such batch.
(4) The name and post office address of the person requesting certification of such batch.
(5) Be accompanied by any label or labeling intended to be used.
80.31 Certification.
(a) If the Commissioner determines, after such investigations as he considers to be necessary, that:
(1) A request submitted in accordance with sec. 80.21 appears to contain no untrue statement of a material fact;
(2) Such color additive conforms to the specifications and any other conditions set forth therefor in parts 81 and 82 of this chapter.
(3) The batch covered by such request otherwise appears to comply with the regulations in this chapter, the Commissioner shall issue to the person who submitted such request a certificate showing the lot number assigned to such batch and that such batch, subject to the terms, conditions, and restrictions prescribed by part 74, 81, and 82 of this chapter, is a certified batch.
(b) If the Commissioner determines, after such investigation as he considers to be necessary, that a request submitted in accordance with sec. 80.21, or the batch of color additive covered by such request, does not comply with the requirements prescribed by paragraph (a) of this section for the issuance of a certificate, the Commissioner shall refuse to certify such batch and shall give notice thereof to the person who submitted such request, stating his reasons for refusal. Any person who contests such refusal shall have an opportunity for a regulatory hearing before the Food and Drug Administration pursuant to part 16 of this chapter.
80.32 Limitations of certificates.
(a) If a certificate is obtained through fraud or misrepresentation of a material fact, such certificate shall not be effective, and a color additive from the batch on which such certificate was issued shall be considered to be from a batch that has not been certified in accordance with the regulations in this part. Whenever, the Commissioner learns that any certificate has been obtained through fraud or material misrepresentation, he shall notify the holder of the certificate that it is of no effect.
(b) If between the time a sample of color additive accompanying a request for certification is taken and the time a certificate covering the batch of such color additive is received by the person to whom it is issued, any such color additive becomes changed in composition, such certificates shall not be effective with respect to such changed color additive and such changed color additive shall be considered to be from a batch that has not been certified in accordance with the regulations in this part.
(c) If at any time after a certificate is received by the person to whom it is issued any color additive from the batch covered by such certificate becomes changed in composition, such certificate shall expire with respect to such changed color additive. After such expiration, such color additive shall be considered to be from a batch that has not been certified in accordance with this part; except that such color additive shall not be so considered when used for coloring a food, drug, or cosmetic, or for the purpose of certifying a batch of a mixture in which such color additive was used as an ingredient, or for use in preparing a batch of a mixture for which exemption from certification has been authorized, if such change resulted solely from such use.
d) A certificate shall expire with respect to any color additive covered thereby if the package in which such color additive was closed for shipment or delivery is opened. After such expiration such color additive shall be considered to be from a batch that has not been certified, except that such color additive shall not be so considered when the package is opened;
(1) and such color additive is used, subject to the restrictions prescribed by paragraphs (f), (g), and (h) of this section, in coloring a food, drug, or cosmetic;
(2) for the purpose of certifying a batch made by repacking such color;
(3) for the purpose of certifying a batch of a mixture in which such color is used as an ingredient; or
(4) for the purpose of preparing a batch of a mixture for which exemption from certification has been authorized; or
(5) when the package is reopened solely for repackaging by the person to whom such certificate was issued.
(e) A certificate shall not be effective with respect to a package of color additive and such color additive shall be considered to be from a batch that has not been certified if such package is shipped or delivered under a label which does not bear all words, statements, and other information required by sec. 70.25 of this chapter to appear thereon.
(f) A certificate shall not be effective with respect to a package of color additive, and such color additive shall be considered to be from a batch that has not been certified if:
(1) Such package has not been sealed in accordance with sec. 70.20 of this chapter.
(2) Such package has been sealed in accordance with sec. 70.20 of this chapter and the seal has been broken, intentionally or accidentally, unless such seal has been broken for the purpose of using color additive in accordance with sec. 80.38, or, such package has been opened by a duly authorized representative of the Administration or Department in the performance of his official duties, and he has immediately resealed the package in conformance with sec. 70.20 of this chapter.
(g) A certificate shall not be effective with respect to a package of color additive and such color additive shall be considered to be from a batch that has not been certified if such color additive is used in any manner other than that for which it was certified.
(h) When the listing or the specifications for a color additive are revoked or amended, the final order effecting the revocation or amendment may specify, in addition to its own effective date, a date on which all certificates for existing batches and portions of batches of such a color additive theretofore issued under such revoked or amended regulations shall cease to be effective; and any such lots of the color additive shall be regarded as uncertified after the date specified unless a new certificate can be and is obtained in conformance with the new regulations. When a certificate thus ceases to be effective for a color additive, any certificates previously issued for a color additive mixture containing that color additive shall cease to be effective on the same date. Use of such color additive or color additive mixture after such specified date without the new certificate in preparing foods, drugs, or cosmetics will result in such food, drugs, or cosmetics being adulterated. When a certified color additive has been used in food, drugs, or cosmetics and the status of the color additive is thereafter changed by amendment or revocation of its listing or specification regulations, such food, drugs, and cosmetics will not be regarded as adulterated by reason of the use of such color additive, unless the hazard to health is such that existing stocks of the foods, drugs, or cosmetics cannot be safely used, in which cases findings to that effect will be made and regulations appropriate for such special cases will be issued.
80.34 Authority to refuse certification service.
(a) When it appears to the Commissioner that a person has:
(1) Obtained, or attempted to obtain, a certificate through fraud or misrepresentation of a material fact.
(2) Falsified the records required to be kept by sec. 80.39; or
(3) Failed to keep such records, or to make them available, or to accord full opportunity to make inventory of stocks on hand or otherwise to check the correctness of such records, as required by sec. 80.39; or
(4) Refused to permit duly authorized employees of the Food and Drug Administration free access to all manufacturing facilities, processes, and formulae involved in the manufacture of color additives and intermediates from which such color additives are derived; he may immediately suspend certification service to such person and may continue such suspension until adequate corrective action has been taken.
(b) Any person who contests suspension of service shall have an opportunity for a regulatory hearing before the Food and Drug Administration pursuant to part 16 of this chapter.
80.35 Color additive mixtures; certification and exemption from certification.
(a) Color additive mixtures to be certified. Any color additive mixture that contains one or more straight colors listed in part 74 of this chapter, together with any diluents listed in such subparts for use with such straight colors, shall be certified if intended for use in foods, drugs, or cosmetics, or in coloring the human body, as the case may be, subject to any restriction prescribed in parts 70 and 71 of this chapter.
(b) Color additive mixtures exempted from certification. A color additive mixture prepared from a previously certified batch of one or more straight colors, with or without any diluent that has been listed in part 73 of this chapter for use in mixtures, shall be exempt from batch certification if the straight color used has not changed in composition in any manner whatsoever since its certification and if it is simply mixed with the approved diluents for exempt mixtures. The label of such color additive mixtures shall not bear the lot number assigned by the Food and Drug Administration to the certified straight color components, but shall bear the manufacturer's control number through which the history of the straight color can be determined.
(c) Additions to the list of diluents. A person requesting additions to the list of diluents authorized for the purposes described in paragraphs (a) and (b) of this section shall submit a petition in accordance with the provisions of sec. 71.1 of this chapter. Each such petition shall be accompanied by the fee prescribed in sec. 70.19 of this chapter, unless there is an advance deposit to be used for prepayment of such fees.
Note: The provisions of sec. 80.35 with respect only to diluents for use in cosmetic color additive mixtures were stayed, until a regulation is effected listing safe diluents for cosmetic use, including cosmetics which color the human body, 29 FR 18495, Dec. 29, 1964.
80.37 Treatment of batch pending certification.
Immediately after the sample that is to accompany a request for certification of a batch of color additive is taken, the batch shall be:
(a) Stored in containers of such kind as to prevent change in composition.
(b) Held under the control of the person requesting certification until certified.
(c) Marked, by labeling or otherwise, in a manner such that there can be no question as to the identity of the batch and no question that it is not to be used until the requested certificate has been issued.
80.38 Treatment of batch after certification.
(a) Immediately upon notification that a batch of color additive has been certified, the person requesting certification thereof shall identify such batch, by labeling, with the certified lot number.
(b) The person requesting certification shall maintain storage in such manner as to prevent change in composition until such batch has been packaged and labeled as required by sec. 70.20 and sec. 70.25 of this chapter, except that the person requesting certification may use such color additive for the purpose of coloring a food, drug, or cosmetic.
80.39 Records of distribution.
(a) The person to whom a certificate is issued shall keep complete records showing the disposal of all the color additive from the batch covered by such certificate. Upon the request of any officer or employee of the Food and Drug Administration or of any other officer or employee acting on behalf of the Secretary of Health and Human Services, such person, at all reasonable hours until at least 2 years after disposal of all such color additive, shall make such records available to any such officer or employee, and shall accord to such officer or employee full opportunity to make inventory of stocks of such color additive on hand and otherwise to check the correctness of such records.
(b) The records required to be kept by paragraph (a) of this section shall show:
(1) Each quantity used by such person from such batch and the date and kind of such use.
(2) The date and quantity of each shipment or delivery from such batch, and the name and post office address of the person to whom such shipment or delivery was made.
(c) The records required to be kept by paragraph (a) of this section shall be kept separately from all other records.