News & Events
PDUFA V: Medical Innovation, Jobs, and Patients
Statement of
Janet Woodcock, M.D.
Director, Center for Drug Evaluation and Research
Food and Drug Administration
Department of Health and Human Services
Before the
Subcommittee on Health
Committee on Energy and Commerce
U.S. House of Representatives
July 7, 2011
INTRODUCTION
Mr. Chairman and Members of the Subcommittee, I am Dr. Janet Woodcock, Director of the Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration (FDA or the Agency), which is part of the Department of Health and Human Services (HHS). Thank you for the opportunity to be here today to discuss the fifth reauthorization of the Prescription Drug User Fee Act (PDUFA), also referred to as “PDUFA V,” and other efforts underway at the Agency to continue access to innovative new medicines and to address the continuing challenges of a global marketplace.
Background on PDUFA
FDA considers the timely review of the safety and effectiveness of new drug applications (NDAs) and biologics license applications (BLAs) to be central to the Agency’s mission to protect and promote the public health. Prior to enactment of PDUFA in 1992, FDA’s drug review process was not very predictable and was relatively slow compared to other countries. As a result of concerns expressed by both industry and patients, Congress enacted PDUFA, which provided the added funds through user fees that enabled FDA to hire additional reviewers and support staff and upgrade its information technology systems. At the same time, FDA committed to complete reviews in a predictable time frame. These changes revolutionized the drug approval process in the United States and enabled FDA to speed the application review process for new drugs and biologics without compromising the Agency’s high standards for demonstration of safety, efficacy, and quality of new drugs prior to approval.
PDUFA Achievements
PDUFA has produced significant benefits for public health, providing patients faster access to over 1,500 new drugs and biologics since enactment in 1992, including treatments for cancer, infectious diseases, neurological and psychiatric disorders, and cardiovascular diseases.
Figure 1. U.S. Share of New Active Substances (NAS) First Launched on the World Market

As shown in Figure 1, the United States now leads the world in the first introduction of new active drug substances.[1] According to researchers at Tufts Center for the Study of Drug Development, the time required for the FDA approval phase of new drug development has been cut by 60 percent since the enactment of PDUFA,[2] from an average of 2.0 years for the approval phase at the start of PDUFA to an average of 1.1 years today.
FDA often hears claims that the United States approves new drugs less quickly than its foreign counterparts, particularly the European Medicines Agency (EMA). While we are not in competition with other countries, we recognize it is our public health duty to approve drugs as quickly and safely as possible. A recent article in the journal Health Affairs[3] compared cancer drugs approved in the United States and Europe between 2003 and 2010. Of the 35 cancer drugs approved by FDA or the EMA from October 2003 to December 2010, FDA approved 32—in an average time of 261 days. EMA approved only 26 of these products, and its average time was 373 days. All 23 cancer drugs approved by both agencies during this period were marketed first in the United States.
Increased resources provided by user fees have enabled FDA to provide a large body of technical guidance to industry that clarified the drug development pathway for many diseases, and to meet with companies during drug development to provide critical advice on specific development programs. In the past five years alone, FDA has held over 7,000 meetings within a short time after a sponsor’s request. Innovations in drug development are being advanced by many new companies as well as more established ones, and new sponsors may need, and often seek, more regulatory guidance during development. In FY 2009, more than half of the meetings FDA held with companies at the early investigational stage and midway through the clinical trial process were with companies that had no approved product on the U.S. market.
Improvements in the efficiency of the drug review process and the quality of new drug applications is evident in the trends toward greater first-cycle approvals for novel drugs, known as “priority” new molecular entities (NMEs). A first-cycle approval means that the product application is approved after the initial, complete FDA review, rather than entering another cycle of FDA questions. Importantly, first-cycle approvals bring innovative drugs with new benefits to patients sooner. The average first-cycle approval rate for priority NMEs has increased from 46 percent in PDUFA I to 68 percent to date in PDUFA IV. First-cycle approval rates have also increased for standard NMEs from an average of 30 percent in PDUFA I to 38 percent to date in PDUFA IV.
PDUFA provides FDA with a source of stable, consistent funding that has made possible our efforts to focus on promoting innovative therapies and help bring to market critical products for patients. Under PDFUA IV, FDA agreed to aim to review priority NMEs more quickly—6 months vs. 10 months for standard drugs. Priority NMEs represent the truly innovative medicines generally targeted at severe illnesses with few or no available therapeutic options. FDA reviewers give these drugs priority attention throughout development, working with sponsors to determine the most efficient way to collect the data needed to provide evidence of safety and effectiveness.
Two recent examples of innovative products given priority review are Victrelis (boceprevir) and Incivek (telapravir), two new drugs for the treatment of hepatitis C. These drugs were approved this past May following the first FDA review cycle and are significant advances for patients suffering with hepatitis C. The Centers for Disease Control and Prevention estimate that about 3.2 million people in the United States have chronic hepatitis C. Most liver transplants performed in the United States are due to progressive liver disease caused by hepatitis C virus infection. Some with hepatitis C will develop cirrhosis of the liver over many years which can lead to liver damage with complications such as bleeding, jaundice (yellowish eyes or skin), fluid accumulation in the abdomen, infections, or liver cancer.
It should be noted that FDA assesses the benefit-risk of new drugs on a case-by-case basis, considering the degree of unmet medical need and the severity and morbidity of the condition the drug is intended to treat. This approach has been critical to increasing patient access to new drugs for cancer and rare and other serious diseases, where existing therapies have been few and limited in their effectiveness. FDA followed this approach in its recent approval of Yervoy (ipilimumab) to treat patients with late-stage (metastatic) melanoma. Yervoy is the first therapy approved by FDA to clearly demonstrate that patients with metastatic melanoma live longer by taking this treatment.
Melanoma is the leading cause of death from skin disease. An estimated 68,130 new cases of melanoma were diagnosed in the United States during 2010, and about 8,700 people died from the disease last year, according to the National Cancer Institute. Late-stage melanoma is devastating, with very few treatment options for patients, none of which previously prolonged a patient’s life. All patients in the study had stopped responding to other FDA-approved or commonly used treatments for melanoma. Patients who received Yervoy alone, or in combination with an experimental vaccine, lived an average of about 10 months, while those who received only the experimental vaccine lived an average of 6.5 months. However, Yervoy also poses a risk of serious side effects, including severe to fatal autoimmune reactions in 12.9 percent of patients treated with Yervoy. FDA decided that the benefits of Yervoy outweighed its risk, especially considering that no other melanoma treatment has been shown to prolong a patient’s life.
PDUFA funds help support the use of existing mechanisms in place to expedite the approval of certain promising investigational drugs and also to make them available to the very ill as early in the development process as possible, without unduly jeopardizing the patients’ safety.
One such program is accelerated approval. In 1992, FDA instituted the accelerated approval process, which allows earlier approval of drugs that treat serious diseases and that fill an unmet medical need based on a surrogate endpoint that is reasonably likely to predict clinical benefit, but is not fully validated to do so. A surrogate endpoint is a marker—a laboratory measurement, or physical sign—that is used in clinical trials as an indirect or substitute measurement that represents a clinically meaningful outcome, such as survival or symptom improvement. The use of a surrogate endpoint can considerably shorten the time to approval. Approval of a drug based on an unvalidated surrogate endpoint is given on the condition that post-marketing clinical trials verify the anticipated clinical benefit. Over 60 critical products have been approved under accelerated approval since the program was established.
While the best means of providing access to useful medical treatments for all Americans is to approve drugs proven to be safe and effective, FDA also recognizes circumstances in which there is public health value in making products available prior to marketing approval. A promising but not yet fully evaluated treatment may sometimes represent the best choice for individuals with serious or life-threatening diseases who lack a satisfactory therapy.
FDA allows for access to investigational products through multiple mechanisms including clinical trials, single patient INDs and treatment protocols. Clinical trials are the best mechanism for a patient to receive an investigational drug because they provide a range of patient protections and benefits and they maximize the gathering of useful information about the product, which benefits the entire patient population. However, there are times when an individual cannot enroll in a clinical trial. In these cases, the patient may gain access to an investigational therapy through one of the alternative mechanisms.
Drug Safety Activities
In parallel with improvements in the drug review process, FDA has increased its focus on drug safety, including implementing the Food and Drug Administration Amendments Act of 2007 (FDAAA). In FDAAA, Congress authorized additional user fees totaling $225 million for the five years of PDUFA IV reauthorization to enhance drug safety activities. FDAAA also provided FDA with important post-market safety authorities. Under FDAAA, FDA was given the ability to require post-marketing studies and clinical trials to address important drug safety questions. Between the enactment of FDAAA on September 27, 2007, and June 1, 2011, FDA has required sponsors to conduct approximately 375 post-marketing studies or trials to address important drug safety questions that could not be addressed before the drug was approved. For example, FDA has required post-marketing clinical trials for Visicol and OsmoPrep (oral sodium phosphate bowel cleansing preparations) to assess the risk of developing acute kidney injury in patients undergoing bowel cleansing using these products. FDA is tracking the conduct of these studies and will take enforcement action if, without good cause, the studies are not conducted in a timely manner.
FDAAA also gave FDA the authority to require safety labeling changes based on new safety information identified after a drug is on the market. FDA has used its new authority to require sponsors to place important new safety information onto their drug labels quickly, in some cases using this authority to require changes to the labeling of all members of a class of drugs. For example, FDA required labeling changes for the Long Acting Beta Agonist (LABA) products to include a new warning in the labels that these products should not be used alone in the treatment of asthma. Although these medicines play an important role in helping some patients control asthma symptoms, they also pose an increased risk of asthma exacerbations, hospitalizations and death. For this reason, their use should be limited whenever possible.
FDAAA also provided FDA with authority to manage risks associated with marketed drug products through required Risk Evaluation and Mitigation Strategies (REMS). FDA has been using this new authority judiciously. For example, FDA required a REMS for Caprelsa (vandetanib), the drug approved to treat medullary thyroid cancer. The approved REMS required a Medication Guide, a communication plan, and elements to assure safe use, including prescriber education and enrollment and pharmacy enrollment and training to mitigate certain cardiovascular risks and sudden death that can occur in some patients. Without this REMS, the benefits of Caprelsa would not have been considered to outweigh the risks, and the drug could not have been approved.
In addition, FDA has implemented other important drug safety initiatives under FDAAA, including, for example, initiating systematic reviews of the safety of marketed drugs 18 months after approval; conducting regular screening of the adverse event reporting system database and posting quarterly reports of new safety information or potential signals of serious risks identified from that screening; and developing an active post-market drug safety surveillance capability under the “Sentinel” initiative.
Challenges for the Current Program
Although we can report many important successes with the current PDUFA program, new challenges have also emerged that offer an opportunity for further enhancement. For example, while new FDAAA process requirements have strengthened drug safety, they have put strains on the review process time frames agreed to, as well as on post-market review activities, which compete for the same resources. In addition, there has been a significant increase in the number of foreign sites included in clinical trials to test drug safety and effectiveness, and an increase in the number of foreign facilities used in manufacturing new drugs for the U.S. market. While foreign sites can play an important role in enabling access to new drugs, the need to travel much further to conduct preapproval inspections for clinical trials and manufacturing sites overseas has created additional challenges for completion of FDA’s review within the existing PDUFA review performance goals, while at the same time trying to communicate with sponsors to see if identified issues can be resolved before the review performance goal date.
Despite these challenges, FDA has maintained strong performance in meeting the PDUFA application review goals, with the exception of a dip in FY 2008-09, when staff resources were shifted to ensure timely implementation of all the new FDAAA provisions that affected activities in the new drug review process. This is shown in Figure 2.
Figure 2. CDER PDUFA Application Review Performance
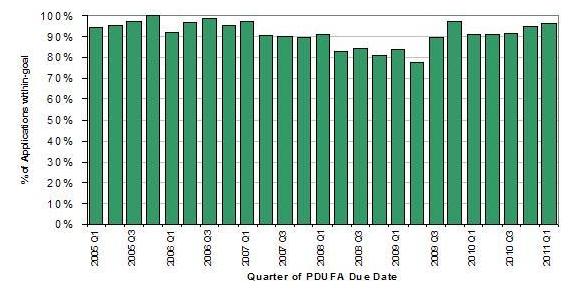
However, FDA wants to meet not only the letter (i.e., PDUFA goal dates), but also the spirit of the original PDUFA goal—speeding patient access to drugs shown to be safe and effective for the indicated uses. Therefore, the Agency is working towards getting more products approved in the first review cycle by trying to identify factors leading to first-cycle approval.
PDUFA Reauthorization
In PDUFA IV, Congress directed FDA to take additional steps to ensure that public stakeholders, including consumer and patient organizations, would have adequate opportunity to provide input to any program enhancements for PDUFA V. Congress directed the Agency to hold an initial public meeting and then to meet with public stakeholders periodically while conducting negotiations with regulated industry, to hear their views on the reauthorization and their suggestions for changes to the PDUFA performance goals. PDUFA IV also required that minutes from negotiation sessions held with industry be made public.
Based on a public meeting held in April 2010, input from a public docket, and the Agency’s own internal analyses of program challenge areas FDA developed a set of potential proposed enhancements for PDUFA V and in July 2010, began negotiations with industry and parallel discussions with public stakeholders. These discussions were concluded in May 2011 and the enhancements are under internal review. We plan to hold a public meeting in the fall to solicit comments on proposed recommendations and then transmit the final recommendations to Congress in January 2012.
We are very pleased to report that the enhancements for PDUFA V under consideration address many of the top priorities identified by public stakeholders, the top concerns identified by industry, and the most important challenges identified within FDA. I will briefly summarize the enhancements under consideration.
A. A Review Program for New Drug Applications (NDA,) New Molecular Entities (NME), and Original Biologics License Applications (BLA)
FDA’s existing review performance goals for priority and standard applications—6 and 10 months respectively—were established in 1997. Since that time, additional requirements in the drug review process have made those goals increasingly challenging to meet, particularly for more complex applications like NME NDAs and original BLAs. FDA also recognizes that increasing communication between the Agency and sponsors during the application review has the potential to increase efficiency in the review process.
To address the desire for increased communication and greater efficiency in the review process, an enhancement being considered in FDA’s review program for NME NDAs and original BLAs in PDUFA V would include pre-submission meetings, mid-cycle communications, and late-cycle meetings between FDA and sponsors for these applications. To accommodate this increased interaction during regulatory review, FDA’s review clock would begin after the 60-day administrative filing review period. The impact of these modifications on the efficiency of drug review for this subset of applications would be assessed during PDUFA V.
B. Enhancing Regulatory Science and Expediting Drug Development
The following five enhancements focus on regulatory science and expediting drug development. Regulatory science is the science of developing and applying new tools, standards and approaches to assess the safety, effectiveness, quality and performance of FDA-regulated products.
1. Promoting Innovation Through Enhanced Communication Between FDA and Sponsors During Drug Development
FDA recognizes that timely interactive communication with sponsors can help foster efficient and effective drug development. In some cases, a sponsor’s questions may be complex enough to require a formal meeting with FDA, but in other instances, a question may be relatively straightforward such that a response can be provided more quickly. However, our review staff’s workload and other competing public health priorities can make it challenging to develop an Agency response to matters outside of the formal meeting process.
This enhancement involves a dedicated drug development communication and training staff, focused on improving communication between FDA and sponsors during development. This staff will be responsible for identifying best practices for communication between the Agency and sponsors, training review staff, and disseminating best practices through published guidance.
2. Methods for Meta-analysis
A meta-analysis typically attempts to combine the data or findings from multiple completed studies to explore drug benefits and risks and, in some cases, uncover what might be a potential safety signal in a premarket or post-market context. However, there is no consensus on best practices in conducting a meta-analysis. With the growing availability of clinical trial data, an increasing number of meta-analyses are being conducted based on varying sets of data and assumptions. If such studies conducted outside FDA find a potential safety signal, FDA will work to try to confirm—or correct—the information about a potential harm that will create uncertainty for patients and health professionals. To do this, FDA must work quickly to conduct its own meta-analyses to include publicly available data and the raw clinical trial data submitted by drug sponsors that would typically not be available to outside researchers. This is resource-intensive work, and often exceeds the Agency’s on-board scientific and computational capacity, causing delays in FDA findings that prolong public uncertainty.
PDUFA V enhancements being considered include the development of a dedicated staff to evaluate best practices and limitations in meta-analysis methods. Through a rigorous public comment process, FDA would develop guidance on best practices and the Agency’s approach to meta-analysis in regulatory review and decision-making.
3. Biomarkers and Pharmacogenomics
Pharmacogenomics and the application of qualified biomarkers have the potential to decrease drug development time by helping to demonstrate benefits, establish unmet medical needs, and identify patients who are predisposed to adverse events. FDA provides regulatory advice on the use of biomarkers to facilitate the assessment of human safety in early phase clinical studies to support claims of efficacy and to establish the optimal dose selection for pivotal efficacy studies. This is an area of new science where the Agency has seen a marked increase in sponsor submissions to FDA. In the 2008-2010 period, the Agency experienced nearly a four-fold increase in this type of review work.
PDUFA V enhancements being considered include augmenting the Agency’s clinical, clinical pharmacology, and statistical capacity to adequately address submissions that propose to utilize biomarkers or pharmacogenomic markers. The Agency would also hold a public meeting to discuss potential strategies to facilitate scientific exchanges on biomarker issues between FDA and drug manufacturers.
4. Use of Patient-reported Outcomes (PRO)
Assessments of study endpoints known as patient-reported outcomes (PROs) are increasingly an important part of successful drug development. PROs measure treatment benefit or risk in medical product clinical trials from the patients’ point of view. They are critical in understanding the drug benefits and harm from the patients’ perspective. However, PROs require rigorous evaluation and statistical design and analysis to ensure reliability to support claims of clinical benefit. Early consultation between FDA and drug sponsors can ensure that endpoints are well-defined and reliable. However, the Agency does not have the capacity to meet the current demand from industry.
PDUFA V enhancements being considered include an initiative to improve FDA’s clinical and statistical capacity to address submissions involving PROs and other endpoint assessment tools, including providing consultation during the early stages of drug development. In addition, FDA will convene a public meeting to discuss standards for PRO qualification, new theories in endpoint measurement, and the implications for multi-national trials.
5. Development of Drugs for Rare Diseases
FDA’s oversight of rare disease drug development is complex and resource intensive. Rare diseases are a highly diverse collection of disorders, their natural histories are often not well-described, only small population sizes are often available for study, and they do not usually have well-defined outcome measures. This makes the design, execution, and interpretation of clinical trials for rare diseases difficult and time consuming, requiring frequent interaction between FDA and drug sponsors. If recent trends in orphan designations are any indication, FDA can expect an increase in investigational activity and marketing applications for orphan products in the future.
Another PDUFA V enhancement being considered includes FDA facilitation of rare disease drug development by issuing relevant guidance, increasing the Agency’s outreach efforts to the rare disease patient community, and providing specialized training in rare disease drug development for sponsors and FDA staff.
C. Enhancing Benefit-Risk Assessment
FDA has been exploring an effort to develop an enhanced structured approach to benefit-risk assessments that accurately and concisely describes the benefit and risk considerations in the Agency’s drug regulatory decision-making. Part of FDA’s decision-making lies in thinking about the context of the decision—an understanding of the condition treated and the unmet medical need. Patients who live with a disease have a direct stake in the outcome of the drug review process. The FDA drug review process could benefit from a more systematic and expansive approach to obtaining the patient perspective on disease severity and the potential gaps or limitations in available treatments in a therapeutic area.
PDUFA V enhancements include expanded implementation of FDA’s benefit-risk framework in the drug review process, including holding public workshops to discuss the application of frameworks for considering benefits and risks that are most appropriate for the regulatory setting. FDA would also conduct a series of public meetings between its review divisions and the relevant patient advocacy communities to review the medical products available for specific indications or disease states that will be chosen through a public process.
D. Enhancement and Modernization of the FDA Drug Safety System
The enhancements being considered for PDUFA V include two post-market, safety-focused initiatives.
1. Standardizing REMS
FDAAA gave FDA authority to require REMS when FDA finds that a REMS is necessary to ensure that the benefits of a drug outweigh its risks. Some REMS are more restrictive types of risk management programs that include elements to assure safe use (ETASU). These programs can require such tools as prescriber training or certification, pharmacy training or certification, dispensing in certain health care settings, documentation of safe use conditions, required patient monitoring, or patient registries. ETASU REMS can be challenging to implement and evaluate, involving cooperation of all segments of the health care system. Our experience with REMS to date suggests that the development of multiple individual programs has the potential to create burdens on the health care system and, in some cases, could limit appropriate patient access to important therapies.
PDUFA V enhancements being considered would initiate a public process to explore strategies and initiate projects to standardize REMS with the goal of reducing burden on practitioners, patients, and others in the health care setting. Additionally, FDA would conduct public workshops and develop guidance on methods for assessing the effectiveness of REMS and the impact on patient access and burden on the health care system.
2. Using the Sentinel Initiative to Evaluate Drug Safety Issues
FDA’s Sentinel Initiative is a long-term program designed to build and implement a national electronic system for monitoring the safety of FDA-approved medical products. FDAAA required FDA to collaborate with federal, academic, and private entities to develop methods to obtain access to disparate data sources and validated means to link and analyze safety data to monitor the safety of drugs after they reach the market, and activity also known as “active post-market drug safety surveillance.” FDA will use user fee funds to conduct a series of activities to determine the feasibility of using Sentinel to evaluate drug safety issues that may require regulatory action, e.g., labeling changes, post-marketing requirements, or post-marketing commitments. This may shorten the time it takes to better understand new or emerging drug safety issues. PDUFA V enhancements would enable FDA to initiate a series of projects to establish the use of active post-market drug safety surveillance in evaluating post-market safety signals in population-based databases. By leveraging public and private health care data sources to quickly evaluate drug safety issues, this work may reduce the Agency’s reliance on required post-marketing studies and clinical trials.
E. Required Electronic Submissions and Standardization of Electronic Application Data
The predictability of the FDA review process relies heavily on the quality of sponsor submissions. The Agency currently receives submissions of original applications and supplements in formats ranging from paper-only to electronic-only, as well as hybrids of the two media. The variability and unpredictability of submitted formats and clinical data layout present major obstacles to conducting a timely, efficient, and rigorous review within current PDUFA goal time frames. A lack of standardized data also limits FDA’s ability to transition to more standardized approaches to benefit-risk assessment and impedes conduct of safety analyses that inform FDA decisions related to REMS and other post-marketing requirements.
PDUFA V enhancements would include a phased-in requirement for standardized, fully electronic submissions during PDUFA V for all marketing and investigational applications. Through partnership with open standards development organizations, the Agency would also conduct a public process to develop standardized terminology for clinical and nonclinical data submitted in marketing and investigational applications.
F. User Fee Increase for PDUFA V
Implementing the PDUFA enhancements being considered would add $40.4 million to the estimated PDUFA user fee revenue amount in FY 2012. This translates to a modest 6 percent increase, and a total estimated base of $712.8 million in FY 2013. [4]
G. PDUFA V Enhancements for a Modified Inflation Adjuster and Additional Evaluations of the Workload Adjuster
In calculating user fees for each new fiscal year, FDA adjusts the base revenue amount by inflation and workload as specified in the statute. PDUFA V enhancements being considered include a modification to the inflation adjuster to accurately account for changes in its costs related to payroll compensation and benefits as well as changes in non-payroll costs. In addition, FDA would continue evaluating the workload adjuster that was developed during the PDUFA IV negotiations to ensure that it continues to adequately capture changes in FDA’s workload.
Additional Initiatives to Encourage Development of New Therapies
Both FDA and the pharmaceutical industry are facing economic and scientific challenges in drug development. Industry is facing a “patent-cliff,” where many of the most profitable brand-name products will face generic competition for the first time. Although this generic competition will benefit consumers by bringing down the cost of these medicines, their manufacturers will lose large sources of profit. Industry must also navigate changing reimbursement rules and increasing expenses from clinical trials. Only a profitable industry can continue to fund the research and development necessary to find new cures.
Although the NDA/BLA approval phase of drug development (the phase in which FDA plays the biggest role) is reported to have the highest success rate of any phase of drug development, it is critical to our public health mission that we work with industry and other stakeholders to take steps to reduce uncertainty and increase the success in the other phases of drug development. To promote the development of innovative new therapies, FDA is working on advancing our scientific base, even apart from the resources contained in the PDUFA V enhancements being considered. With so much at stake for public health, FDA has made advances in regulatory science a top priority. The Agency is both supporting mission critical science at FDA and exploring a range of new partnerships with the National Institutes of Health and academic institutions to develop the science needed to maximize advances in biomedical research and bring the development and assessment of promising new therapies into the 21st century. With this effort, FDA is poised to support a wave of innovation to transform medicine and save lives.
For example, FDA is working to improve the science behind certain clinical trial designs. Recent advances in two clinical trial designs—called non-inferiority and adaptive designs—have required FDA to conduct more complex reviews of clinical trial protocols and new marketing applications. Improving the scientific bases of these trial designs should add efficiency to the drug review process, encourage the development of novel products, and speed new therapies to patients.
FDA has also taken steps to facilitate the development and approval of safe and effective drugs for Americans with rare diseases. Therapies for rare diseases—those affecting fewer than 200,000 people in the United States—represent the most rapidly expanding area of drug development. Although each disease affects a relatively small population, collectively rare diseases affect about 25 million Americans. Approximately one-third of the NMEs and new biological products approved in the last five years have been drugs for rare diseases. Because of the small numbers of patients who suffer from each disease, FDA often allows non-traditional approaches to establishing safety and effectiveness. For example, FDA recently approved Myozyme (alglucosidase alfa) for the treatment of Pompe Disease, which is a rare genetic disease resulting in progressive skeletal and respiratory muscle weakness caused by an accumulation of glycogen (a carbohydrate). About 1,000-2,000 patients in the United States suffer from Pompe Disease, of which only a few hundred are infants. In infants, the disease can be rapidly fatal due to respiratory failure. FDA approved this drug in April 2006, based on the results of a single, pivotal study in 18 patients.
In March 2010, FDA approved Carbaglu (carglumic acid) for the treatment of N-acetylglutamate synthase (NAGS) deficiency, the rarest of the Urea Cycle Disorders, which are diseases that lead to elevated ammonia levels in the blood and cause seizures, poor muscle tone, respiratory distress, coma, and even death. NAGS deficiency affects fewer than 10 patients in the United States at any given time. FDA approved this drug based on a case series in 23 patients.
The Challenges Posed by Globalization
In addition to reauthorizing PDUFA, FDA is also committed to meeting challenges posed by increased globalization. When President Franklin Delano Roosevelt established the modern FDA in 1938, the percentage of food and medical products imported into the United States was minimal. Today up to 40 percent of the drugs Americans take are manufactured outside our borders, and up to 80 percent of the active pharmaceutical ingredients in those drugs comes from foreign sources. Last month, FDA published a special report, “Pathway to Global Product Safety and Quality,” our global strategy and action plan that will allow us to more effectively oversee the safety of all products that reach U.S. consumers in the future. Over the next decade, FDA will transform itself from a domestic Agency, operating in a globalized world, to a truly global Agency fully prepared for a regulatory environment in which product safety and quality knows no borders. To achieve this transformation, the Agency is developing an international operating model that relies on improved information sharing and gathering, data-driven risk analytics, and the smart allocation of resources through partnerships.
We engage in international drug standards development and harmonization efforts, and last year we conducted more foreign inspections than ever before in our history. We also just established a new Office of Drug Integrity, Security, and Recalls, which specifically focuses on drug quality issues such as counterfeiting, economically motivated adulteration, cargo theft, and other supply chain threats and vulnerabilities.
New regulatory authorities may help ensure that we can hold industry accountable for the security and integrity of their supply chains and the quality control systems they use to produce drugs for the American people. In our increasingly complex and globalized world, additional authorities could be important tools to help support FDA’s efforts to protect the safety of imports and the health of our citizens.
CONCLUSION
PDUFA IV expires on September 30, 2012, and FDA is ready to work with you to ensure timely reauthorization of this critical program. If we are to sustain and build on our record of accomplishment, it is critical that the reauthorization occur seamlessly without any gap between the expiration of the old law and the enactment of PDUFA V. Thank you for your contributions to the continued success of PDUFA and to the mission of FDA. I am happy to answer questions you may have.
_______________________________________________________________
[1] Source: Scrip NCE Review/Scrip Yearbook/Scrip Magazine (1982 -2005), PharmaProjects R&D Annual Review (2006-2009).
[2] Milne, Christopher-Paul (2010). PDUFA and the Mission to Both Protect and Promote Public Health [PowerPoint slides]. Presentation at the FDA PDUFA Public Meeting, Rockville, MD.
[3] “Despite Criticism Of The FDA Review Process, New Cancer Drugs Reach Patients Sooner In The United States Than In Europe,” Samantha A. Roberts, Jeff D. Allen, and Ellen V. Sigal, Health Affairs, June 2011.
[4] The FY 2012 estimated user fee amount is $672.4 million. The exact amount will be determined when we have the final-year workload data for PDUFA IV. That number would be used to calculate the exact fee amounts for FY 2013, the first year of PDUFA V.







