Medical Devices
FDA AND NIOSH Public Health Notification: Oxygen Regulator Fires Resulting from Incorrect Use of CGA 870 Seals
(You are encouraged to copy and distribute this information)
Updated: June 19, 2006
Dear Colleagues:
We are updating the Public Health Notification of April 24, 2006 alerting you to the danger of fires at the interface of oxygen regulators and cylinder valves because of incorrect use of CGA 870 seals, and to point out an important precaution you can take to avoid such fires.
This update clarifies the FDA and NIOSH recommendation on the use of sealing- type washers (reusable, metal-bound rubber seal) and crush-type gaskets (single use, not reusable, usually Nylon ®) with oxygen regulators. We believe that this new language will alleviate concerns around the proper use of both types of seals.
Background
FDA has received 12 reports in which regulators used with oxygen cylinders have burned or exploded, in some cases injuring personnel. Some of the incidents occurred during emergency medical use or during routine equipment checks. FDA and NIOSH believe that improper use of gaskets/washers in these regulators was a major factor in both the ignition and severity of the fires, although there are likely other contributing factors.
Two types of washers, referred to as CGA 870 seals, are commonly used to create the seal at the cylinder valve / regulator interface: The type recommended by many regulator manufacturers is a metal-bound elastomeric sealing washer that is designed for multiple use applications. The other common type, often supplied free-of-charge with refilled oxygen cylinders, is a plastic (usually Nylon ®) crush gasket suitable for single use applications.
When used more than once, the Nylon ® crush gaskets require higher torque than the elastomeric sealing washers in order to seal the cylinder valve / regulator interface, and if they are used again, they require more torque with each successive use. The cylinder valve / regulator connection is designed to be hand-tightened. If the crush gaskets are re-used, the need for increased torque may require using a wrench or other hand tool, which can deform the crush gasket and damage the cylinder valve and regulator. This can result in leakage of oxygen past the cylinder valve seat and across the nylon crush gasket. According to a forensic analysis supported by FDA and NIOSH, “flow friction” caused by this leakage of compressed oxygen across the surface of the crush gasket may produce enough thermal energy to spontaneously ignite the nylon gasket material.
Recommendations
FDA and NIOSH recommend that plastic crush gaskets never be reused, as they may require additional torque to obtain the necessary seal with each subsequent use. This can deform the gasket, increasing the likelihood that oxygen will leak around the seal and ignite.
The following general safety precautions should also be taken to avoid explosions, tank ruptures and fires from oxygen regulators.
- Always “crack” cylinder valves (open the valve just enough to allow gas to escape for a very short time) before attaching regulators in order to expel foreign matter from the outlet port of the valve.
- Always follow the regulator manufacturer’s instructions for attaching the regulator to an oxygen cylinder.
- Always use the sealing gasket specified by the regulator manufacturer.
- Always inspect the regulator and CGA 870 seal before attaching it to the valve to ensure that the regulator is equipped with only one clean, sealing- type washer (reusable metal-bound rubber seal) or a new crush-type gasket (single use, not reusable, typically Nylon ®) that is in good condition.
- Always be certain the valve, regulator and gasket are free from oil or grease. Oil or grease contamination is widely known to contribute to ignition in oxygen systems.
- Tighten the T-handle firmly by hand, but do not use wrenches or other hand tools that may over-torque the handle.
- Open the post valve slowly. If gas escapes at the juncture of the regulator and valve, quickly close the valve. Verify the regulator is properly attached and the gasket is properly placed and in good condition. If you have any questions or concerns contact your supplier.
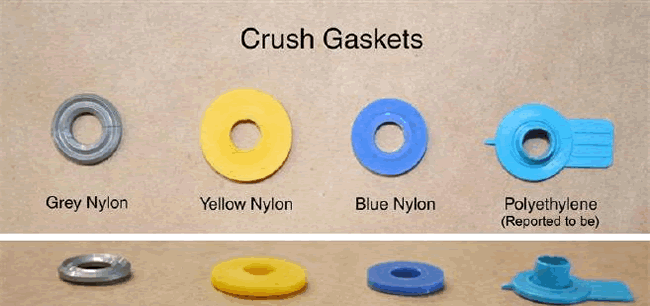
Figure 1 : Examples of crush gaskets available for CGA 870 type medical post valves

.Figure 2: Examples of some sealing washers available for CGA 870 Style medical post valves.
Reporting to FDA
FDA requires hospitals and other user facilities to report deaths and serious injuries associated with the use of medical devices. If you suspect that a reportable adverse event was related to the use of medical gas equipment, you should follow the reporting procedure established by your facility.
We also encourage you to report adverse events related to medical gas equipment that do not meet the requirements for mandatory reporting. You can report these directly to the device manufacturer. You can also report to MedWatch, the FDA’s voluntary reporting program. You may submit reports to MedWatch by phone at 1-800-FDA-1088; by FAX at 1-800-FDA-0178; by mail to MedWatch, Food and Drug Administration, 5600 Fishers Lane, Rockville, MD 20852-9787; or online.
Getting More Information
FDA Medical Device Public Health Notifications are available on the Internet. You can also be notified through email each time a new Public Health Notification is added to our web page. To subscribe, visit: http://service.govdelivery.com/service/subscribe.html?code=USFDA_39.
Sincerely yours,
Daniel Schultz, MD
Director
Center for Devices and Radiological Health
Food and Drug Administration
Nancy Stout, Ed.D.
Director, Division of Safety Research
National Institute for Occupational Safety and Health
Centers for Disease Control and Prevention
If you have questions about this Notification, please contact FDA's Division of of Small Manufacturers, International and Consumer Assistance (DSMICA) by e-mail at dsmica@fda.hhs.gov or by phone at 1-800-638-2041 or 301-796-7100
If you have questions about this Notification, please contact FDA's Division of of Small Manufacturers, International and Consumer Assistance (DSMICA) by e-mail at dsmica@fda.hhs.gov or by phone at 1-800-638-2041 or 301-796-7100