Thermostabilized enzyme created for biofuels production
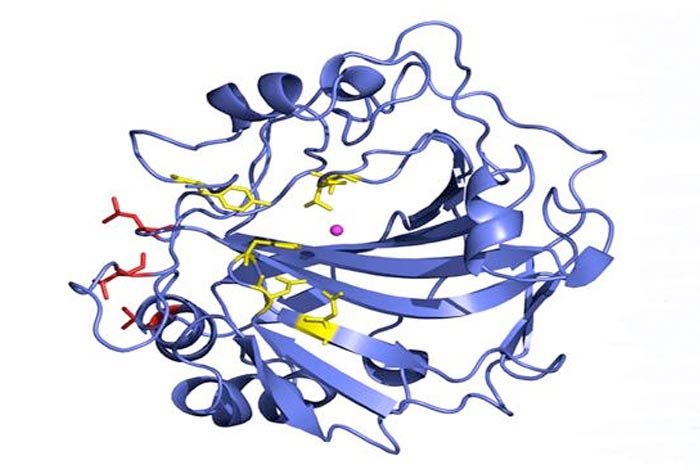
Ribbon diagram of human carbonic anhydrase II in blue. The red ball-and-stick section depicts the three surface amino acid residues that were mutated to confer thermal stability. The active site zinc (Zn) and its amino acid residues are shown in magenta and yellow, respectively.
Contact
- Bioenergy and Environmental Science
- Zoë Fisher
- (505) 665 4105
Thermostabilized enzyme created for biofuels production
Zoë Fisher of Bioenergy and Environmental Science (B-8) and collaborators from the University of Florida have created an enzyme that has the ability to capture carbon with much greater efficiency. The paper, which describes thermally stabilized human carbonic anhydrase II (HCA II), was published in the journal Protein Engineering: Design and Selection and featured on the cover. Carbonic anhydrases (CAs) are ubiquitous enzymes that regulate the interconversion of carbon dioxide (CO2) and bicarbonate. Within the human body, carbonic anhydrases control the acid-base balance in the blood and enable transport of CO2 out of tissue. HCA II is one of the fastest enzymes ever studied. These enzymes might serve as biocatalysts for carbon sequestration and biofuel production if they could function under harsh industrial conditions. Therefore, the researchers developed HCA II variants with enhanced thermostability while retaining high solubility and catalyticactivity.
Enhancing enzyme stability
Although carbonic anhydrase operates with near perfect efficiency at room temperature, the enzyme quickly “dies” as temperatures increase. If this enzyme could tolerate higher temperatures while maintaining or improving its normal activity, then it could be used as a biocatalyst for carbon capture from noxious flue gas, a rich source of CO2 produced in coal-fired power plants. However, flue gas is largely inaccessible for biofuel applications due to the high operating temperatures. The researchers made an experimental breakthrough for using carbonic anhydrase in the hot environments that are relevant in biofuel applications. The team identified specific amino acids on the outer surface of HCA II that, when mutated, give enhanced physical properties to this enzyme. The scientists created a variant of HCA II in which they substituted a cluster consisting of three hydrophobic (repelling water) amino acid residues with more polar/charged ones. The substitutions enhance the thermal stability of HCA II and have no significant effect on the active site structure or the catalytic rate.
Structural changes improve stability, boost catalysis
The research team determined the structural and functional effects of these mutations. A gain in surface hydrogen bonding contributes to thermal stability while retaining proper active site geometry and electrostatics to sustain catalytic efficiency. The scientists next introduced a set of active site mutations, modifying the active site water network to boost catalytic activity. This combination of designed mutations creates HCA II variants with improved thermal stability and enzymatic activity.
Mary Jo Waltman and David Fox of B-8, and Paul Langan (formerly of LANL, currently at Oak Ridge National Laboratory) also contributed to the research. The DOE Office of Biological Environmental sponsors the Protein Crystallography Station, and an LDRD Early Career Grant funded the Los Alamos research. The work supports the Lab’s Energy Security mission area and the Materials for the Future science pillar.