Archive
FDA Unveils New Section of the FDA Web site for Recalls Data and Other Agency Data Sets
Materials Available from FDA Public Webinar About the “510(k) Premarket Review Process” and the “Use of Science in Regulatory Decision Making” Draft Reports
Did you miss the FDA webinar discussion on the draft reports about the 510(k) process and the use of science in regulatory decisions?
The webinar was discussed in a August 26, 2010 blog post. An audiofile of the discussion, along with the background materials, is available on the CDRH website. You can listen to the discussion here.
FDA is soliciting comments from the public on the draft reports via www.regulations.gov, docket number FDA-2010-N-0348, until October 4, 2010.
Afia Asamoah, Transparency Initiative Coordinator
Upcoming Event: FDA Basics Webinar on Generic Drugs, Monday, September 27th, 1:00 p.m. ET
Did you know that generic drugs are safe and effective alternatives to brand name drugs? Did you know that generic drugs can reduce the cost of prescription drugs for both consumers and the government?
As part of FDA Basics, FDA is hosting a webinar where you can learn more. The featured speaker Robert West, a supervisory pharmacist in the Office of Generic Drugs in FDA’s Center for Drug Evaluation and Research (CDER), will give an overview of generic drug regulation. After the presentation, there will be an opportunity to ask questions.
The free 30 minute webinar will be held on Monday, September 27, at 1:00 p.m. ET.
Sign in early, since there are a limited number of spots available for the webinar. Materials from the webinar will also be made available on the FDA Web site.
Click here for more information about the webinar, including instructions about how to join the webinar.
Afia Asamoah, Transparency Initiative Coordinator
Coming Soon: Newly Redesigned FDA Basics Main Page
It’s been almost 9 months since the FDA Basics section of the FDA website launched. After collecting web traffic data during this time, we decided to make some minor changes to the main page in hopes of improving your experience.
The changes include:
- Cleaner look-and-feel
- Easier access to the major content areas, videos, and webinars
- Restructured slideshow
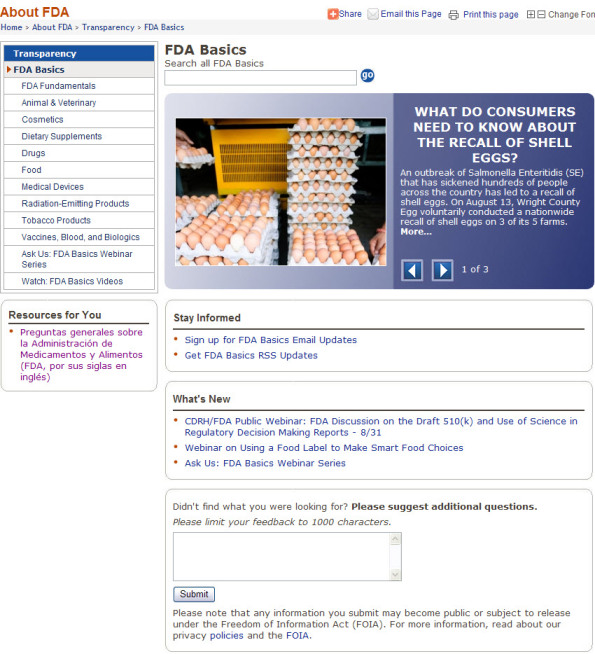
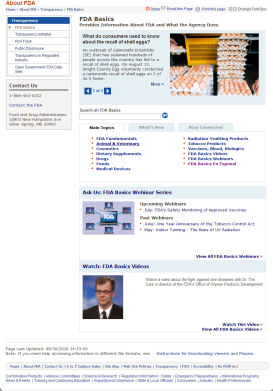
The newly redesigned FDA Basic main page will launch later this month. In the meantime, see below for images of the current main page (1st picture) and the new main page (2nd picture). Click the images below to see the full size image.
Post a comment below to let us know what you think.
Afia Asamoah, Transparency Initiative Coordinator
Coming Soon: New Look to the FDA Transparency Blog
You may notice some changes and a few new features on the FDA Transparency Blog soon. FDA is planning to move the blog to a new hosting platform in the next few weeks.
After the move, you will be able to tag blog posts with a 1-5 ‘star’ rating and easily view the top rated posts with a single click. In addition, you will be able to easily print and share posts via email in the form of new buttons at the bottom of each blog post. We also plan to add similar buttons that will allow you to share posts with your friends on popular social networking sites like Twitter and Facebook.
Afia Asamoah, Transparency Initiative Coordinator
FDA-TRACK: Stay Informed on Agency-Wide Program Performance by Subscribing to FDA-TRACK Web Updates
In April, FDA launched a web resource called FDA-TRACK that allows the public to track the agency's progress on a range of measures, including whether the agency is hitting its inspection targets and whether it is hitting its targets for completing reviews of product applications (see April 7 blog post).
Now, you can receive free email notifications when measures tracked on FDA-TRACK are updated by subscribing to "FDA-TRACK Updates." "FDA-TRACK Updates" will send you an email alerting you when the performance data on FDA-TRACK are updated. The subscription will allow you to choose how often you would like to recieve updates. You can cancel at any time.
Thank you for your feedback thus far. We are working to incorporate suggestions and continuously improve the site. Please continue to visit and let us know what you think. Suggested changes and additional feedback can be submitted to FDATRACK@fda.hhs.gov.
Malcolm Bertoni, Assistant Commissioner for Planning at FDA