Archive
Check It Out: Newly Redesigned FDA-TRACK Dashboard Pages
FDA’s performance management system, FDA-TRACK, allows the public to follow the agency’s progress on a range of measures (see April 7 blog post).
We redesigned FDA-TRACK! It has been a little over one year since the launch of the FDA-TRACK website. After collecting web traffic data during this time, we decided to make some changes to the dashboard pages in hopes of improving your experience. The changes include:
- Cleaner look-and-feel;
- Charts and options to overlay related measures;
- XML downloads; and
- “FDA-TRACK Indices” by program area, for easier access navigation to specific content.
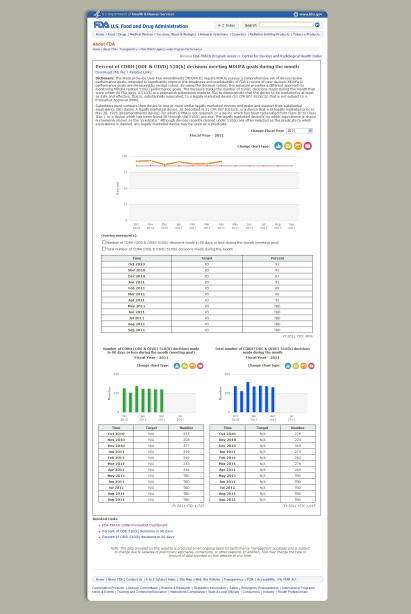
Check out these before and after shots:
Before:
After:
Please visit any of the FDA-TRACK dashboard pages to see these changes.
The US and Mexico Share Approaches on Food Safety
In June, I had the opportunity to lead a delegation of food safety officials from the Food and Drug Administration to meet with our Mexican counterparts. The trip was part of a larger, proactive strategy to reach out to stakeholders, both domestic and foreign, to explain the background and implementation strategies for the new Food Safety Modernization Act (FSMA) and importantly, to listen to issues raised by stakeholders. Following Canada, Mexico is the largest exporter of foods to the United States. It was an exciting opportunity to meet with Mexican officials, not only to provide outreach on our new law, but also to gain a better understanding of Mexican food safety interests and challenges, and to identify areas for collaboration to further ensure the safety of foods for our respective populations.
Throughout the discussions, our team was impressed with the level of agreement on overarching principles and strategies to assure foods are safe for our respective populations. Like us, Mexico has embraced food safety as a priority and is in the process of establishing new mandatory food safety regulations, including produce safety regulations. Some of the key themes that were emphasized throughout our discussions were consumer protection, science- based standards, the need for importer controls, the importance and role of third- party certification. Throughout our discussions, I felt that I was indeed hearing many of the principles embodied in FSMA, such as the importance of prevention, the need to establish strong partnerships, a robust import program, and an effective program of risk-based inspections. In addition, there was a common recognition of the importance of making sure new regulations consider trade impacts, and the importance of transparency in rulemaking. Our strong alignment on key principles was both gratifying and extremely encouraging.
We had a whirlwind schedule. Highlights are as follows:
Our FDA delegation was welcomed to Mexico by Honorable John D. Feeley, U.S. Chargé d’Affaires to Mexico, and Mr. James H. Williams, Acting Deputy Chief of Mission, at the U.S. Embassy in Mexico City. The FDA delegation also met with representatives of U.S. agencies at the U.S. embassy in Mexico from Foreign Agricultural Services, Foreign Commercial Service, Animal and Plant Health Inspection Service; and the State Department Economic Section. Embassy officials were very helpful in sharing their perspectives on FSMA and potential cooperative activities with Mexico. Last year, FDA opened its new office in Mexico City. We were delighted to see our staff integrated into US Embassy activities and representing the agency in a stellar manner with our regulatory counterparts.
Next, we had an informative meeting with Mexican Government agencies involved in food safety. Mexican Government agencies represented included: Secretariat of Agriculture – Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación / Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA); Secretariat of Health – Comisión Federal para la Protección contra Riesgos Sanitarios (COFEPRIS); Secretariat of Economy – Secretaria de Economía; Secretariat of Foreign Affairs – Secretaria de Relaciones Exteriores; and Secretariat of the Environment- Ambiente y Recursos Naturales (which is the agency in charge of CONAGUA which plays an essential role in helping to ensure the quality of water used in farming and agriculture). Later in the day, we had a discussion with SENASICA to learn more and discuss their latest proposal for the revision of the FDA-SENASICA Cantaloupe MOU, which is scheduled to expire in October, 28, 2011. The parties agreed to further discuss possible opportunities for the enhancement of the MOU including possible enhanced collaboration on technical and operational protocols, exchange of regulatory information, as well as on food safety research.
We had the pleasure of having lunch with representatives of Mexican academic institutions including: National Autonomous University of Mexico (Universidad Nacional Autónoma de México); National Polytechnic Institute (National Instituto Politecnico); The Autonomous University of Puebla (Benemérita Universidad Autónoma de Puebla); and the Postgraduate College (Colegio de Postgraduados). The luncheon emphasized the important contribution of academia to the regulatory process on innovation and research, not only in food safety science but also in pubic health, political science, and business management.
One of the most dynamic parts of the day was a joint FDA, SENASICA and COFEPRIS roundtable with Mexican industry and trade organizations. Industry and trade expressed their concerns, views, and suggestions on FSMA. Participants discussed Mexico’s inspection protocols and FDA’s inspection procedures and import protocols at the border. We addressed questions related to produce safety preventive controls.
Following the meeting with industry, I participated in a press conference with senior officials from SENASICA and COFEPRIS and Mexican news media during which we were able to reinforce common messages on food safety.
We had a wonderful, productive day in Mexico. We acknowledged and indeed celebrated our common commitment to food safety.
Michael R. Taylor, Deputy Commissioner for Foods